Press release
Growth in OEM Healthcare Boosts Custom Electrode Placement Pads Manufacturer China
As global healthcare systems increasingly move toward personalization, remote monitoring, and non-invasive therapy solutions, demand for specialized components has increased across the medical device supply chain. At this critical juncture in healthcare systems transformation lies the role of custom electrode placement pads manufacturers China(https://www.careboohealth.com/product/tens-unit-replacement-pads-16pcs-premium-thickened-reusable-self-adhesive-electrode-pads-for-ems-muscle-stimulator-massager-2x2-16-pcs-2/). Custom electrode pads manufacturers provide OEM healthcare brands with flexible design, scalable production capacity and regulatory-ready solutions - and Careboo has emerged as a technology-driven healthcare company providing innovative sleep health, pain management and physical therapy applications to support OEM healthcare ecosystem.Industry Outlook: Extending OEM Healthcare and Tech-Driven Care Solutions
The global healthcare industry is currently experiencing a structural transformation driven by demographic shifts, chronic conditions and an increase in sleep and pain disorders. Healthcare delivery models have expanded beyond hospitals into homes, wellness centers and fitness environments - leading to the explosive growth in OEM (Original Equipment Manufacturing) healthcare as global brands search for reliable partners who can develop customized, compliant components at scale.
One of the industry's most noteworthy trends is the increase in non-invasive therapy technologies. Electrical stimulation, red light therapy, heat and cold therapy and pressure-based solutions have become widely adopted to relieve pain relief, muscle recovery, sleep improvement and rehabilitation. Such technologies rely heavily on precision components-such as electrode placement pads-that directly interact with humans for safety, comfort and performance purposes.
Sleep health has emerged as a key growth driver. Rising rates of insomnia, snoring, sleep apnea and related breathing disorders has driven both consumers and healthcare providers to look for user-friendly solutions to these disorders. Sleep monitoring devices, snoring intervention systems and neuromuscular stimulation products have expanded quickly across both clinical and consumer markets; creating demand for custom electrodes and placement pads tailored specifically to anatomical requirements, therapy protocols or device designs.
Personalization has emerged as a prominent industry trend. Original Equipment Manufacturer (OEM) healthcare brands no longer rely on off-the-shelf components; instead they require tailored electrode shapes, materials, adhesion levels and conductivity profiles that address specific use cases--whether jaw muscle stimulation for snoring reduction, targeted pain relief or neuromuscular activation during rehabilitation. China, with its advanced medical manufacturing infrastructure and engineering talent has emerged as an essential hub for such customization at scale.
Regulatory Strength and Certifications: Building Trust
As OEM healthcare manufacturing increasingly crosses borders, ensuring regulatory compliance with multiple regulatory frameworks simultaneously is increasingly crucial. Certifications therefore become not just credentials but strategic enablers of global market access.
Careboo operates within an extremely regulated environment and aligns its manufacturing and quality management systems to internationally recognized standards. Products and production processes designed for European markets comply with CE standards to ensure safety, performance and compliance with directives and regulations; additionally, alignment with MDR (Medical Device Regulation) shows our company's dedication to meeting even the strictest medical device regulations - an imperative commitment particularly pertinent when using devices involving electrical stimulation or direct skin contact.
Quality management at Careboo is further strengthened through compliance with ISO 13485, the global quality standard for medical device quality systems. This certification ensures that its design, production and post-market processes follow a structured risk-based approach with emphasis on traceability, consistency and continuous improvement - an advantage for OEM partners as ISO 13485 compliance reduces regulatory risk while speeding time to market.
Careboo's US operations place equal emphasis on regulatory readiness. Careboo's expertise with FDA requirements, such as experience with 510(k), assists OEM customers targeting the world's largest medical device market. Compliance with both agencies ensures products meet stringent safety and effectiveness criteria that support legal marketed devices.
Careboo's certifications represent a comprehensive compliance framework designed to support their role within the global OEM healthcare ecosystem, facilitating seamless collaboration with international brands, contract manufacturers and healthcare providers while guaranteeing end users receive safe, reliable products that exceed expectations.
OEM Healthcare Growth and the Emergence of Custom Electrode Solutions
China's rapid expansion in OEM healthcare manufacturing has spurred rapid development of customized electrode placement pads. As medical devices become more specialized, electrodes become essential components to therapeutic effectiveness, user comfort, and clinical outcomes.
Careboo specializes in sleep health, pain management and physical therapy services. Their solutions have produced notable success in addressing snoring by targeting jaw muscle activity and respiratory soft tissue weakness; using electrical pulse technology and targeted stimulation their solutions help users reduce and stop snoring while improving sleep quality for users and their families.
Careboo's technologies combine electrical pulses with pressure, heat, cold, light and other physical factors - including pressure, heat, cold light and light therapy - for integrated approaches that address pain relief, vitality enhancement relaxation muscle recovery joint strain sports injuries post exercise rehabilitation rehabilitation needs and post workout rehab rehabilitation. Electrode placement pads play a crucial role in providing precise therapy solutions in each application and ultimately providing consistent comfortable therapy treatments.
OEM healthcare growth has spurred manufacturers to create electrodes that are both functional and user-friendly. Careboo has developed electrode pads with ergonomic design principles and high-grade materials to conform naturally to your body, maintain stable contact, and minimize skin irritation during repeated use - this focus on user experience is especially critical as more medical devices enter home environments or consumer wellness settings.
As global brands outsource development and manufacturing to specialist providers, Careboo's role extends beyond production. Careboo works alongside OEM customers on design optimization, material selection and customization services - helping transform clinical concepts into market-ready products that can scale. This collaborative approach is reflective of an emerging trend within Chinese manufacturers who have transformed from cost-focused suppliers into innovation-focused solution providers.
Looking Ahead
OEM healthcare's growth is revolutionizing the global medical device landscape, with custom electrode placement pads playing an instrumental role. As demand for personalized, noninvasive healthcare solutions increases at home, manufacturers that successfully combine regulatory compliance, technological know-how and ergonomic design will become even more influential.
Careboo's longstanding dedication to sleep health, pain management and advanced physical therapy technologies positions the company to capitalize on global healthcare trends while adding real value for OEM partners worldwide. By providing high-grade materials and cutting-edge technology solutions that comply with certified quality systems, Careboo enables healthcare devices that enhance global quality of life.
For more information about Careboo and its healthcare solutions, please visit https://careboohealth.com/ -
Telephone
+86-13760204436
Mailbox
qd0755@163.com
Address
No. 7 Chuangye Road Dongshan Village, Qishi Town, Dongguan City
Careboo focuses on sleep health and healthcare related products. Careboo has long been committed to the research, development, and technology of various sleep disorders and pain disorders.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Growth in OEM Healthcare Boosts Custom Electrode Placement Pads Manufacturer China here
News-ID: 4345739 • Views: …
More Releases from CAREBOO CO., LIMITED

China CAREBOO Respiratory Device Manufactor CE/FDA Highlights Compliance Strengt …
Careboo, a China CE/FDA respiratory device manufacturer(https://www.careboohealth.com/product-category/snoring/), is delighted to join Arab Health 2026 at Dubai Exhibition Centre as an exhibitor and take advantage of this premier platform to demonstrate their latest advancements in sleep health and respiratory therapy, emphasizing their rigorous commitment to international safety standards and therapeutic efficacy for an international audience.
The Global Sleep Health Horizon: Industry Prospects and Trends
The global market for sleep apnea and respiratory devices…
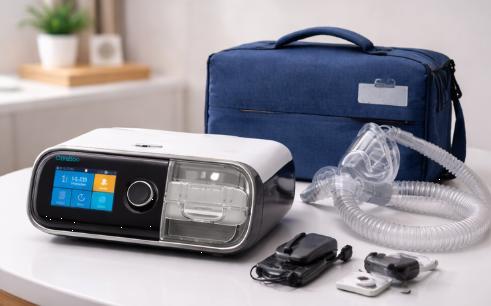
CAREBOO Air Breathing Equipment Manufacturer China Introduces New Respiratory Sy …
As global respiratory awareness and the prevalence of sleep-related breathing disorders increase, so has the demand for advanced, safe, and patient-centric respiratory systems. CAREBOO air breathing equipment manufacturer China is emerging as a leader in this space-offering high-quality solutions across hospitals, sleep clinics, and homecare settings. Its innovative range combines advanced technology, rigorous regulatory compliance, and patient-centric design, setting benchmarks in respiratory therapy while unveiling its latest innovations at the…

MDR Compliant Breathing Device Manufactor China vs Low-Cost Alternatives
Global healthcare standards have reached a crucial turning point, prompting an unprecedented need for high-performance respiratory therapy solutions. At Careboo, a pioneering sleep health and advanced medical technology company, care is taken in serving as a premier MDR compliant breathing device manufactor China(https://www.careboohealth.com/product-category/snoring/) while prioritizing clinical safety. By adhering to the rigorous EU 2017/745 Medical Device Regulation and prioritizing clinical safety and regulatory alignment over standard sleep aids, Careboo provides…

How CE Approved Auto Ventilator Wholesale China Meets Rising Clinical Requiremen …
CE approved auto ventilator wholesale China(https://www.careboohealth.com/product-category/snoring/) has become an indispensable sourcing channel for hospitals, distributors, and healthcare providers looking for high-quality devices that balance safety with performance and scalability. Leading this transformation is Careboo, an healthcare technology company dedicated to improving sleep health through innovation, compliance, and patient-centric design.
Emergence of Clinical Demand for Automatic Ventilators
Since 2008, clinical requirements for automatic ventilators have steadily grown over time. Occlusive Sleep Apnea (OSA),…
More Releases for OEM
OEM Partnership Guide: Working with a Touch-free Automatic Kitchen Garbage Can O …
With increasing global demand for smart home solutions, Sinoware International Ltd, a top provider in household products industry, is pleased to unveil expanded OEM partnership initiatives.
Sinoware has established itself in Jiangmen--China's premier stainless steel industry zone--as an indispensable touch-free automatic kitchen garbage can OEM manufacturer for global brands seeking to incorporate high-tech sanitation solutions into their portfolios.
By combining their decades-old tradition of metal craftsmanship with cutting-edge infrared and…
Revolutionizing OEM Coatings With Sustainable Solutions Trend: A Crucial Influen …
Which drivers are expected to have the greatest impact on the over the oem coatings market's growth?
The surge in requirements from final consumer industries is forecasted to boost the expansion of the OEM coatings market. These coatings, referred to as OEM, are utilized during the integration of other firms' products into the substrate process or application. They prove to be beneficial for a variety of end-user sectors, including automotive and…
OEM Technology Partnerships Launches Brokerage Specializing in 100+ OEM Technolo …
San Francisco, California, USA - February 13, 2025 - OEM Technology Partnerships is thrilled to announce the launch of its specialized brokerage focused on connecting businesses with a comprehensive portfolio of over 100 Original Equipment Manufacturer (OEM) technologies. This new venture is poised to revolutionize how companies access and implement cutting-edge solutions across diverse industries.
Leveraging deep industry expertise and a vast network of OEM partners, OEM Technology Partnerships offers a…
OEM or ODM Watches? What's the Difference?
When searching for a watch manufacturer for your store or watch brand, you may come across the terms OEM and ODM. But do you truly understand the difference between them? In this article, we will delve into the distinctions between OEM and ODM watches to help you better grasp and choose the manufacturing service that suits your needs.
Image: https://www.naviforce.com/uploads/15a6ba3911.png
What's OEM / ODM Watches [https://www.naviforce.com/products/]
OEM (Original Equipment Manufacturer) watches are produced…
OEM Partnership with Extreme Networks
ComputerVault announces an OEM partnership with Extreme Networks and has certified its switches for use with ComputerVault enterprise software to deliver virtual desktop infrastructure (VDI).
Extreme Networks industry leading switches deliver ComputerVault Virtual Desktops at faster than PC speeds in the LAN and WAN.
“ComputerVault is very excited to work with Extreme Networks. Not only are their switches very reliable, but their exceptional performance guarantees a great user experience”, said Marc…
Humidity Measurement Module for OEM Applications
The EE1900 humidity module from E+E Elektronik is optimised for the measurement of relative humidity (RH) or dew point temperature (Td) in climate and test chambers. With outstanding temperature compensation across the working range from -70 °C to 180 °C (-94 °F to 356 °F) and the choice of stainless steel and plastic probes, the module is suitable for a wide range of applications.
High Accuracy in Harsh Environment
The excellent…
