Press release
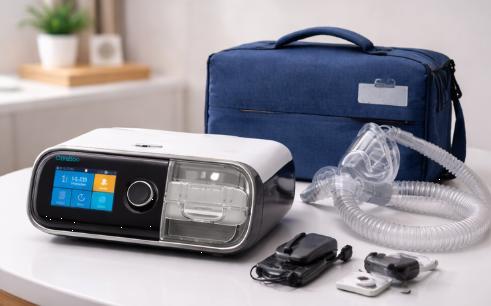
Guide to Working with Reusable TENS Electrodes Manufactory CAREBOO
Brand owners are looking for manufacturing partners who can offer more than just assembly lines. They want a combination of clinical expertise, innovation and regulatory reliability. Careboo has become a leader in the therapeutic technology industry and set new standards for international partnerships. By collaborating with the Reusable TENS electrodes manufacturer CAREBOO(https://www.careboohealth.com/product-category/electric-therapy/tens-units/), entrepreneurs and established medical devices companies can expand their portfolios by offering eco-friendly, high-durability solutions. Careboo provides a seamless transition between concept and global distribution by combining high-end production with research into sleep disorders and pain relief.Part I: Strategic Value of an Electrotherapy Professional Partner
Globally, the "pain relief industry" is the fastest growing and most stable category in the medical devices industry. The barrier to entry for this industry is high because of the complexity of the bio-electrical technology and the requirement for anatomical precision. Brands looking to differentiate themselves can benefit from partnering with Careboo or other factories that specialize in bio-electrical technologies.
Careboo is more than a factory. It is a powerhouse of research dedicated to treating sleep and pain disorders. Careboo's manufacturing philosophy is based on the integration multiple therapeutic modalities. It has years of experience applying physical factors to human healthcare. Careboo, unlike many other manufacturers, has mastered the science behind reusability. This ensures that electrode pads retain high conductivity and adherent for extended periods. Their technology integrates the following:
Electrical pulses & pressure: Effective nerve-blocking treatment and deep muscle reeducation.
Thermal dynamics (Heat and cold): To treat inflammation and chronic joint pain along with electrotherapy.
Light therapy: Use of specific wavelengths for cell repair and enhancement.
This multi-functionality allows brand owners to launch a diverse product line, from TENS units for sport recovery devices to specialized red-light therapy devices, all under one reliable manufacturing umbrella.
Part II: Collaborative R&D - From Sleep Science to Snoring Solutions
Careboo's dual expertise is physical pain relief as well as sleep health, which gives brand owners a distinct advantage. It allows brand owners to tap into the "Restorative Wellness", where pain management is treated as a whole ecosystem. Careboo's monitoring technology is highly effective in determining sleep quality. This data can be used to develop wearable devices that are truly effective.
Careboo's breakthrough progress in addressing snoring is one of the most exciting areas for partnership. Careboo has developed high-performance devices that reduce and stop snoring by researching the mechanics of jaw muscle and soft tissue weakness. Careboo offers brands access to the following:
Technical sophistication: Access advanced sleep monitoring algorithms, stimulation technologies.
Proven effectiveness: Products that are based on "outstanding" results in clinical research.
Ergonomic design: Every device adopts a ergonomic design concept to ensure that the products are not only effective medically but also comfortable throughout the night for users.
Careboo can help your brand provide "high-quality sleeping enjoyment" - a value proposition highly appealing to modern customers who are increasingly sleep-deprived and stressed.
Part III: Operational Roadmap - A Guide to Partnering with Careboo
Working with a professional manufacturer is transparent and efficient. Careboo has optimized its global service model to offer partners an unmatched experience. Here's a guide to a successful partnership.
1. Customized OEM/ODM Products Available for All Budgets
Careboo offers flexible services, whether you need to customize a particular technical parameter or want to white-label a high-quality existing design. Careboo partners can select from a wide range of international products including Sleep Therapy Monitors and Snoring Stop Devices. They can also choose TENS Units or Heating Pads.
2. Material Integrity and Ergonomic Inclusion
Careboo only uses the best materials for its reusable electrodes, including medical-grade hydrogels. Your brand will benefit from the ergonomic design expertise of the factory when you work together. The "touch and feeling" of the product will be in line with its high-tech performance, giving your brand a premium image.
3. Certification and Compliance Streamlined
Medical device manufacturers face a number of challenges when it comes to certification. Careboo's commitment towards healthcare excellence includes the maintenance of standards required for international sales. Careboo can help brand owners reduce the time to market for new therapeutic devices by leveraging its "outstanding research" results and manufacturing success.
4. Global Logistics and Supply Chain Scalability
Careboo's supply chain is scalable and battle-tested. They have sold their products globally, giving brands peace of mind regarding lead times, component reliability and shipping consistency. Careboo is able to scale up or down depending on your needs.
Part IV: Meeting the Diverse Needs of the Modern Consumer
Any brand owner's ultimate goal is to satisfy the end-user. Careboo and you brand will work together to meet different demographics' needs through targeted technology.
The athlete: Provides "vitality enhancement" through TENS units and Red Light units to help muscle strain recovery, performance and performance.
Office Workers Provides pain relief and "relaxation", for joint strains that are caused by an overly sedentary lifestyle.
The Elderly Delivering "better services" through the use of easy-to-use heating pads and sleep monitors that prioritize comfort.
Stop Snoring Devices for Chronic Snorers: Restoring respiratory health and domestic harmony with the latest Snoring Stop Devices.
Careboo's latest technology allows you to offer a "unprecedented experience" for your health-conscious customers. Careboo's emphasis on the physical factors ensures your products are not just "gadgets", but essential tools to a healthier lifestyle.
Your manufacturing partner is the most valuable asset in the highly competitive medical devices and sleep health industries. Working with the Manufacturer of Reusable TENS Electrodes CAREBOO allows you to combine innovative technology with scientific research. Careboo is dedicated to offering people high-quality sleep and pain management solutions that are both sustainable and effective.
It is now time to partner with a manufacturer that values quality, ergonomics and breakthrough results. Reclaim your brand's vitality and give your customers the care they need.
Visit the official Careboo site to learn more about our partnership opportunities, and explore our entire range of therapeutic products.
https://www.careboohealth.com/
Telephone
+86-13760204436
Mailbox
qd0755@163.com
Address
No. 7 Chuangye Road Dongshan Village, Qishi Town, Dongguan City
Careboo focuses on sleep health and healthcare related products. Careboo has long been committed to the research, development, and technology of various sleep disorders and pain disorders.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Guide to Working with Reusable TENS Electrodes Manufactory CAREBOO here
News-ID: 4345720 • Views: …
More Releases from CAREBOO CO., LIMITED

China CAREBOO Respiratory Device Manufactor CE/FDA Highlights Compliance Strengt …
Careboo, a China CE/FDA respiratory device manufacturer(https://www.careboohealth.com/product-category/snoring/), is delighted to join Arab Health 2026 at Dubai Exhibition Centre as an exhibitor and take advantage of this premier platform to demonstrate their latest advancements in sleep health and respiratory therapy, emphasizing their rigorous commitment to international safety standards and therapeutic efficacy for an international audience.
The Global Sleep Health Horizon: Industry Prospects and Trends
The global market for sleep apnea and respiratory devices…
CAREBOO Air Breathing Equipment Manufacturer China Introduces New Respiratory Sy …
As global respiratory awareness and the prevalence of sleep-related breathing disorders increase, so has the demand for advanced, safe, and patient-centric respiratory systems. CAREBOO air breathing equipment manufacturer China is emerging as a leader in this space-offering high-quality solutions across hospitals, sleep clinics, and homecare settings. Its innovative range combines advanced technology, rigorous regulatory compliance, and patient-centric design, setting benchmarks in respiratory therapy while unveiling its latest innovations at the…

MDR Compliant Breathing Device Manufactor China vs Low-Cost Alternatives
Global healthcare standards have reached a crucial turning point, prompting an unprecedented need for high-performance respiratory therapy solutions. At Careboo, a pioneering sleep health and advanced medical technology company, care is taken in serving as a premier MDR compliant breathing device manufactor China(https://www.careboohealth.com/product-category/snoring/) while prioritizing clinical safety. By adhering to the rigorous EU 2017/745 Medical Device Regulation and prioritizing clinical safety and regulatory alignment over standard sleep aids, Careboo provides…

How CE Approved Auto Ventilator Wholesale China Meets Rising Clinical Requiremen …
CE approved auto ventilator wholesale China(https://www.careboohealth.com/product-category/snoring/) has become an indispensable sourcing channel for hospitals, distributors, and healthcare providers looking for high-quality devices that balance safety with performance and scalability. Leading this transformation is Careboo, an healthcare technology company dedicated to improving sleep health through innovation, compliance, and patient-centric design.
Emergence of Clinical Demand for Automatic Ventilators
Since 2008, clinical requirements for automatic ventilators have steadily grown over time. Occlusive Sleep Apnea (OSA),…
More Releases for TENS
Rising Consumer Health Trends Boost CAREBOO EMS & TENS Therapy Device Manufactor …
The global health consciousness is increasing, and the demand for home-based medical devices that are sophisticated has reached new heights. Careboo is a leading innovator in the wellness sector and strategically positioned to satisfy this demand. CAREBOO TENS & EMS therapy device manufacturer(https://www.careboohealth.com/product-category/electric-therapy/) is a hub that produces high-quality medical devices by aligning advanced research and the growing trend toward self-care. Careboo's commitment to integrating digital precision with physical therapy…
Volkswagen plans to cut tens of thousands of employees
Image: https://www.chinashinyfly.com/uploads/W.jpg
Management plans to close at least three local factories and cut tens of thousands of employees to cut operating costs, he said at a staff event at Volkswagen [https://www.chinashinyfly.com/b24f-plastic-quick-connector-for-fuel-system-%cf%867-89-516%e3%80%9e-id6-90-sae-product/] headquarters in Wolfsburg on October 28.
Cavallo said the board had carefully considered the plan and that all German factories could be affected by the closure plan and that other workers who had not been closed would also face pay cuts.…
TENS Machine Market Size 2024 to 2031.
Market Overview and Report Coverage
A Transcutaneous Electrical Nerve Stimulation (TENS) Machine is a device used to provide pain relief through electrical stimulation. The TENS Machine Market is expected to grow at a CAGR of 6.80% during the forecasted period.
The future outlook of the TENS Machine Market is promising due to the increasing prevalence of chronic pain conditions, rising aging population, and growing awareness about non-invasive pain management…
Global TENS Machine Market Research Report
This report studies the global TENS Machine market status and forecast, categorizes the global TENS Machine market size (value & volume) by manufacturers, type, application, and region. This report focuses on the top manufacturers in North America, Europe, Japan, China, and other regions (India, Southeast Asia). The major manufacturers covered in this report HealthmateForever Zewa Omron TruMedic TechCare PurePulse IReliev Therapeutix Geographically, this report studies the top producers and consumers,…
PKB registers tens of thousands of patients with mass registration
Patients Know Best (PKB) - a social enterprise providing the world’s first patient-controlled health records, has introduced ‘mass registration’ enabling patients to sign up to access their health records at scale and with speed.
Following a number of successful trials in partnership with NHS organisations, patients can now sign up for their health record in a number of ways; either by speaking to a member of staff; by using the…
TENS Machine Manufacturing Market Technological breakthroughs 2023
Transcutaneous electrical nerve stimulation (TENS) is the technology in which the electric current generated by the TENS device is used to stimulate the nerves and minimize pains associated with those nerves. TENS technology covers the entire frequencies of transcutaneously utilized currents that are useful in nerve excitation.
TENS machine is a small, portable device which comprises current impulse regulating system and a number of electrode patches (usually one to four). The…
