Press release
Developmental and Epileptic Encephalopathy Treatment Market Poised for Accelerated Expansion, Fueled by Targeted Therapies and a Growing Innovation Pipeline, Says DelveInsight
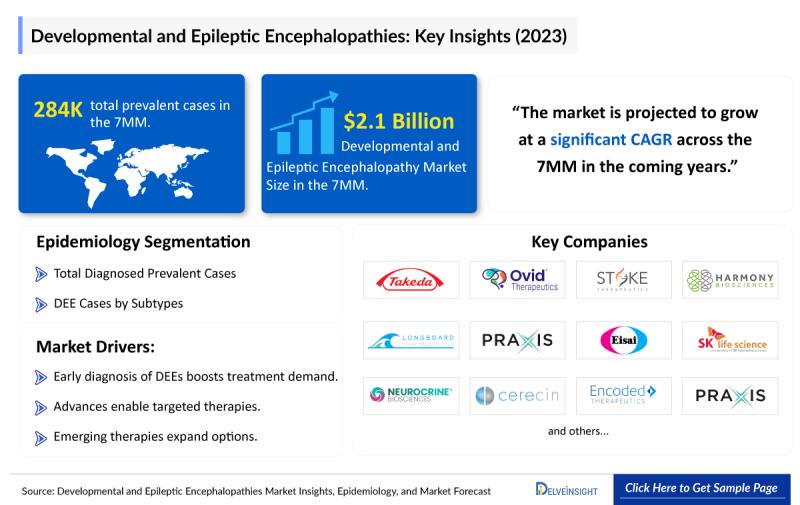
The Developmental and Epileptic Encephalopathy (DEE) treatment market across the seven major markets (7MM) was valued at nearly USD 2.1 billion in 2023 and is expected to register a healthy compound annual growth rate over the forecast period. The United States emerged as the largest contributor, capturing close to 80% of the overall market revenue.The DEE treatment landscape is undergoing a significant transformation as the limitations of traditional antiepileptic drugs become more apparent. Increasing emphasis is being placed on steroid-based therapies, immunomodulators, and precision-driven treatments. Recent regulatory approvals, including EPIDIOLEX and ZTALMY, have broadened available therapeutic options, while FINTEPLA is anticipated to enter additional indications soon, further intensifying market competition.
While currently approved therapies primarily address a limited number of syndromes such as Dravet syndrome, Lennox-Gastaut syndrome, CDKL5 deficiency disorder, and tuberous sclerosis complex, over 20 DEE subtypes remain largely unaddressed. This substantial unmet need represents a major growth opportunity. A strong and diverse development pipeline supported by companies such as Takeda, Eisai, SK Life Science, Stoke Therapeutics, Harmony Biosciences, Longboard Pharmaceuticals, Praxis Precision Medicines, Neurocrine Biosciences, and Encoded Therapeutics continues to reinforce the market's long-term outlook. Nevertheless, challenges including clinical trial failures, high therapy costs, reimbursement hurdles, limited patient access, and a shortage of specialist care may constrain growth to some extent.
DelveInsight's Developmental and Epileptic Encephalopathy Market Insights report delivers a comprehensive evaluation of the disease and treatment landscape, encompassing existing therapies, emerging drugs, and the market share of individual treatments. The report provides an in-depth assessment of historical trends and market forecasts from 2020 to 2034 across the 7MM, covering the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan. In addition, it analyzes treatment pathways, critical growth drivers, market restraints, and unmet medical needs, helping stakeholders identify opportunities and assess the overall commercial potential of the DEE market.
Explore which therapies are expected to capture a significant share of the DEE treatment market @ Developmental and Epileptic Encephalopathy Market Forecast - https://www.delveinsight.com/report-store/developmental-and-epileptic-encephalopathies-dee-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Key Highlights from the Developmental and Epileptic Encephalopathy Market Report
• According to DelveInsight's analysis, the DEE treatment market across the 7MM was valued at approximately USD 2.1 billion in 2023.
• DelveInsight estimates that nearly 235,000 diagnosed prevalent cases of DEE were reported across the 7MM in 2021.
• In December 2025, UCB presented positive efficacy and safety outcomes from the Phase III GEMZ study evaluating adjunctive fenfluramine in pediatric and adult patients with CDKL5 Deficiency Disorder at the American Epilepsy Society (AES) Annual Meeting in Atlanta. The study achieved its primary and key secondary endpoints, demonstrating a statistically significant reduction in countable motor seizure frequency and meaningful improvement on the Clinical Global Impression-Improvement scale versus placebo. UCB intends to submit a regulatory application for fenfluramine in CDKL5 Deficiency Disorder, marking the third DEE indication for which the therapy is being pursued.
• In November 2025, Praxis Precision Medicines announced the successful conclusion of a Type C meeting with the US FDA, resulting in the immediate transition of the EMBRAVE3 registrational study of elsunersen in early-onset SCN2A-related DEE to a single-arm design. All enrolled patients will now receive elsunersen for 24 weeks, followed by an open-label extension.
• In October 2025, Capsida Biotherapeutics received Orphan Drug Designation from the US FDA for CAP-002, its investigational gene therapy targeting DEE caused by STXBP1 mutations. CAP-002 is a first-in-class, intravenously administered gene therapy designed to achieve widespread neuronal expression while minimizing liver exposure and is currently undergoing IND-enabling studies.
• In February 2025, NICE approved FINTEPLA (fenfluramine) for the treatment of Lennox-Gastaut syndrome, making it the first licensed non-cannabis therapy for this condition. This approval represents FINTEPLA's second NHS-funded indication, following its earlier authorization for Dravet syndrome.
• In February 2025, Immedica Pharma AB completed the acquisition of Marinus Pharmaceuticals, gaining ownership of the FDA-approved seizure medication ZTALMY.
• In February 2025, Stoke Therapeutics and Biogen announced a partnership to make zorevunersen, a first-in-class disease-modifying therapy, available to Dravet syndrome patients outside the US, Canada, and Mexico. Following regulatory alignment across the US, Europe, and Japan, a global Phase III registrational study (EMPEROR) is scheduled to begin in Q2 2025, with pivotal data expected in H2 2027.
• In January 2025, Takeda discontinued the soticlestat development program after Phase III SKYLINE and SKYWAY trials in Dravet syndrome and LGS failed to meet primary endpoints. Subsequent discussions with the FDA confirmed that the existing data package was insufficient to support an NDA submission.
• In December 2024, Orion Corporation and Marinus Pharmaceuticals mutually agreed to terminate their European marketing and distribution agreement for ZTALMY, which is approved in Europe for adjunctive treatment of seizures associated with CDKL5 deficiency disorder.
• In December 2024, Longboard Pharmaceuticals initiated a long-term extension study to evaluate the safety, tolerability, and efficacy of LP352 in patients with DEE who completed the LP352-201 study.
• Key companies actively operating in the DEE treatment market include Takeda, Eisai, SK Life Science, Stoke Therapeutics, Harmony Biosciences/Epygenix, Longboard Pharmaceuticals, Praxis Precision Medicines, Neurocrine Biosciences, Bright Minds Biosciences, Cerecin, Encoded Therapeutics, Bloom Science, IAMA Therapeutics, Ultragenyx Pharmaceutical, Neuroene Therapeutics, Regel Therapeutics, Jazz Pharmaceuticals, Biocodex, Zogenix (UCB), Lundbeck, Marinus Pharmaceuticals, Novartis, Aquestive Therapeutics, Supernus Pharmaceuticals, GlaxoSmithKline, Roche, Xenon Pharmaceuticals, Ovid Therapeutics, BioPharm Solutions, and others.
• Promising pipeline candidates include EPX-100, EPX-200, EPX-300, XEN496, LP352, STK-001, JBPOS0101, soticlestat, carisbamate, NBI-921352, NBI-827104, PRAX-562, and additional investigational assets.
• The current DEE treatment landscape in the United States is primarily supported by antiepileptic drugs, Epidiolex/Epidyolex, Fintepla, Afinitor/Votubia, and related therapies.
Developmental and Epileptic Encephalopathy Overview
Developmental and Epileptic Encephalopathy (DEE) encompasses a group of severe neurological disorders marked by early-onset, treatment-resistant seizures and profound developmental impairments. The condition typically manifests during infancy or early childhood and includes syndromes such as Dravet syndrome, Lennox-Gastaut syndrome, and West syndrome. DEE is frequently linked to genetic mutations involving genes such as SCN1A, CDKL5, and STXBP1, leading to abnormal brain development and persistent epileptic activity that result in cognitive, motor, and behavioral deficits.
Common clinical features include frequent seizures and delayed developmental milestones. Diagnosis relies on a combination of genetic testing, electroencephalography (EEG), and neuroimaging techniques such as MRI. Treatment strategies often involve anti-seizure medications, dietary interventions like the diet, and, in select cases, surgical approaches. Despite available options, DEE remains difficult to manage due to poor responsiveness to conventional therapies. Ongoing research is increasingly focused on gene therapies, precision medicine approaches, and next-generation antiepileptic drugs.
Management of DEE requires coordinated multidisciplinary care involving neurologists, genetic specialists, and rehabilitation professionals. Enhancing disease awareness is critical to achieving earlier diagnosis and improving long-term outcomes. Although rare, DEE is a life-altering condition that necessitates continued therapeutic innovation and comprehensive patient support.
Developmental and Epileptic Encephalopathy Epidemiology Segmentation
DelveInsight estimates that approximately 235,000 diagnosed prevalent cases of DEE were recorded across the 7MM in 2021. Among EU4 countries, Germany accounted for the highest number of diagnosed cases during that year.
The epidemiological assessment spans the period from 2020 to 2034 across the 7MM and includes:
• Diagnosed prevalent population of DEE
• Treated population of DEE
Access the full report to explore the factors shaping DEE epidemiology trends @ Developmental and Epileptic Encephalopathy Prevalence - https://www.delveinsight.com/sample-request/developmental-and-epileptic-encephalopathies-dee-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Developmental and Epileptic Encephalopathy Treatment Market
The management of DEE follows general principles used in pediatric epilepsy care, with treatment selection guided by the specific epilepsy syndrome. Anti-seizure medications remain the cornerstone of therapy, although many seizure types associated with DEE are notoriously difficult to control.
FDA-approved therapies for DEE include Epidiolex, Diacomit, Fintepla, Sabril, ZTALMY, Afinitor Disperz/Votubia, Sympazan, Trokendi XR, Onfi, Banzel, Lamictal, and others. In certain pediatric patients, steroid therapies such as ACTH or prednisone have demonstrated clinical benefit. When pharmacological interventions fail, alternative options including vagus nerve stimulation, diet therapy, or epilepsy surgery may be considered. Surgical approaches can range from focal resections to hemispherectomy in carefully selected cases.
Learn more about available DEE treatment strategies @ Developmental and Epileptic Encephalopathy Treatment Market - https://www.delveinsight.com/report-store/developmental-and-epileptic-encephalopathies-dee-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Developmental and Epileptic Encephalopathy Pipeline Therapies and Key Players
• XEN496 - Xenon Pharmaceuticals
• Soticlestat - Takeda / Ovid Therapeutics
• Carisbamate - SK Life Science
• EPX-100 - Epygenix
• NBI-921352 - Neurocrine Biosciences
• STK-001 - Stoke Therapeutics
• NBI-827104 - Neurocrine Biosciences
• JBPOS0101 - BioPharm Solutions
• PRAX-562 - Praxis Precision Medicines
• EPX-200 - Epygenix
• EPX-300 - Epygenix
• LP352 - Longboard Pharmaceuticals
Developmental and Epileptic Encephalopathy Market Dynamics
The DEE market is expected to undergo notable shifts over the coming years. The approval of ZTALMY, the first licensed therapy for CDKL5 deficiency disorder in the US, has significantly strengthened market momentum. Continued R&D efforts are anticipated to address the longstanding lack of approved therapies. Additionally, Epidiolex and Fintepla are expanding their prescriber base, contributing to sustained uptake.
However, market growth is challenged by the complex and heterogeneous nature of rare epileptic syndromes such as LGS, Dravet syndrome, and CDKL5 deficiency disorder, which complicate diagnosis and treatment.
Scope of the Developmental and Epileptic Encephalopathy Market Report
• Study Period: 2020-2034
• Geographic Coverage: 7MM
• Market Size (2023): USD 2.1 billion
• Key Companies: Takeda, Eisai, Neurocrine Biosciences, Stoke Therapeutics, Novartis, Xenon Pharmaceuticals, Marinus Pharmaceuticals, Jazz Pharmaceuticals, and others
• Key Therapies: XEN496, soticlestat, carisbamate, EPX-100, STK-001, PRAX-562, LP352, and more
• Analysis Includes: Market dynamics, competitive intelligence, unmet needs, reimbursement landscape, and strategic insights
Discover more about DEE drugs in development @ Developmental and Epileptic Encephalopathy Clinical Trials and FDA Approvals - https://www.delveinsight.com/sample-request/developmental-and-epileptic-encephalopathies-dee-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Contact Us:
Ankit Nigam
Manager Marketing
info@delveinsight.com
+14699457679
About DelveInsight
DelveInsight is a leading Life Science market research and business consulting company recognized for its off-the-shelf syndicated market research reports and customized solutions to firms in the healthcare sector.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Developmental and Epileptic Encephalopathy Treatment Market Poised for Accelerated Expansion, Fueled by Targeted Therapies and a Growing Innovation Pipeline, Says DelveInsight here
News-ID: 4341441 • Views: …
More Releases from DelveInsight Business Research
Polycythemia Vera Pipeline and Drug Development in 2025: 10+ Therapies and 8+ Co …
DelveInsight's "Polycythemia Vera Pipeline Insight 2025" report delivers an in-depth overview of the Polycythemia Vera pipeline landscape, covering more than 8 companies and 10+ pipeline candidates. The report analyzes both clinical-stage and preclinical assets, offering detailed drug profiles across various stages of development. It also evaluates Polycythemia Vera therapies based on product classification, development stage, route of administration, and molecular category, while additionally spotlighting inactive or discontinued pipeline assets.
Explore the…
Myopic Macular Degeneration market across the 7MM is projected to reach USD 837 …
In 2022, the Myopic Macular Degeneration market across the 7MM was valued at approximately USD 734 million, largely driven by anti-VEGF therapies and standard care. The Myopic Macular Degeneration market is expected to grow gradually through 2034, constrained by a limited pipeline of emerging treatments. Japan accounts for the largest share of diagnosed cases (49%), followed by the United States (27%). Females are disproportionately affected across the 7MM, potentially due…
Netherton syndrome market across the 7MM was valued at approximately USD 25 mill …
The Netherton syndrome market is projected to grow at a 7% CAGR from 2025 to 2034, driven by key regions including the US, EU4, the UK, and Japan. In 2024, around 3,500 diagnosed prevalent cases were reported across the 7MM. Market expansion is primarily supported by advancing diagnostic capabilities, increasing disease awareness, rising diagnosis rates, and a strengthening clinical pipeline led by companies such as Azitra (ATR-12), Quoin Pharmaceuticals (QRX003),…
Sandhoff Disease Pipeline Analysis Highlights Emerging Therapies and Active Indu …
DelveInsight's "Sandhoff Disease Pipeline Insight 2025" report delivers an extensive overview of the Sandhoff Disease pipeline landscape, featuring insights on more than 5 companies and over 5 pipeline candidates. The report analyzes both clinical- and non-clinical-stage drug candidates, offering detailed profiles of Sandhoff Disease pipeline products. It also evaluates emerging therapeutics based on product category, development stage, route of administration, and molecular classification, while additionally highlighting inactive or discontinued pipeline…
More Releases for DEE
Dee Sanders Photography: Capturing Moments, Creating Magic
Image: https://www.abnewswire.com/upload/2025/02/1393acc5fe32c5fda1d96251c531a7e4.jpg
Montgomery, AL - In the dynamic world of visual storytelling, Dee Sanders [https://www.deesandersphotography.com/]Photography has emerged as a beacon of excellence, transforming the way businesses and individuals showcase their stories through captivating imagery. From stunning real estate visuals to heartwarming event captures, this versatile photography studio has become the go-to choice for those seeking to elevate their visual narrative.
Revolutionizing Real Estate Marketing
Dee Sanders Photography has redefined real estate marketing with…
Zee Dee Touch Published Clients' Testimonials
Zee Dee Touch is a trustworthy company in the field of kitchen and bathroom planning and installation. It was founded more than 20 years ago. During that time, Zee Dee Touch successfully finalized numerous projects of planning and installations of both kitchens and bathrooms. This company has just introduced a section with clients' testimonials on its official website with an only aim to share some of the clients' thoughts about…
Zee Dee Touch Celebrated 150th Kitchen Installation
Zee Dee Touch is a firm recognizable for its experienced and dynamic team operating in the field of kitchen planning and installations, residential and commercial renovations, and kitchen and bathroom remodeling. This month it has announced the successful completion of the 150th kitchen renovation project in a private house in Springfield VA. This project is just one in a line of numerous kitchen installation projects when the wishes and needs…
Dee Shackles Market Size, Share, Development by 2024
Global Info Research offers a latest published report on Dee Shackles Market Analysis and Forecast 2019-2025 delivering key insights and providing a competitive advantage to clients through a detailed report. This report focuses on the key global Dee Shackles players, to define, describe and analyze the value, market share, market competition landscape, SWOT analysis and development plans in next few years.
To analyze the Dee Shackles with respect to individual growth…
Zee Dee Touch Focuses on Details in Kitchen Remodeling
Zee Dee Touch makes the overall process of kitchen remodeling easy and hustle-fee to all its present and prospective clients. A team of enthusiastic people employed in this company is constantly at a client’s disposal and goes through every step and detail in the kitchen remodeling process together with the homeowner. This prevents any unnecessary stress and fuss and ensures the satisfaction of the client.
’When it comes to remodeling a…
Zee Dee Touch Launches a Kitchen Planning Checklist
Zee Dee Touch, a premier kitchen remodeling company from Newington VA has launched a practical kitchen planning checklist carefully placed in steps which are easy to understand and accept. It is a technically well-organized and professionally planned list of useful steps which should be followed during the kitchen planning process. Each family which thinks about kitchen design services in Virginia should refer to this originally – conducted manual, in order…