Press release
Global Viral Vector Manufacturing Market Outlook 2025-2032: Strong CAGR of 23.5%, Says Persistence Market Research
The viral vector manufacturing market has emerged as a cornerstone of the modern biopharmaceutical industry, underpinning the rapid evolution of gene therapy, cell therapy, and next-generation vaccines. Viral vectors act as delivery vehicles that transport therapeutic genetic material into target cells, enabling treatments that address diseases at their genetic root rather than merely managing symptoms. As gene therapy transitions from experimental science to commercial reality, the demand for scalable, regulatory-compliant viral vector manufacturing has intensified significantly. Pharmaceutical companies, biotechnology innovators, and contract development and manufacturing organizations (CDMOs) are all investing heavily to expand capacity and adopt advanced production technologies to meet this surging demand.Download Your Free Sample & Explore Key Insights: https://www.persistencemarketresearch.com/samples/26923
In 2025, the global viral vector manufacturing market is valued at approximately US$1.8 billion and is forecast to reach US$8.0 billion by 2032, expanding at a robust compound annual growth rate (CAGR) of 23.5%. This exceptional growth trajectory reflects the convergence of several factors, including accelerated gene therapy approvals, regulatory support for advanced therapies, and increased investment in manufacturing infrastructure. Among virus types, adeno-associated virus (AAV) vectors lead the market due to their favorable safety profile and suitability for in vivo gene therapies, while lentiviral vectors are witnessing the fastest growth driven by the success of CAR-T cell therapies. North America currently dominates the market, accounting for more than half of global revenue, owing to its strong clinical trial ecosystem, extensive funding, and advanced biomanufacturing capabilities.
Key Highlights from the Viral Vector Manufacturing Market Report
• The global viral vector manufacturing market is projected to grow at a CAGR of 23.5% from 2025 to 2032.
• AAV vectors dominate the market with an estimated 45% share in 2025 due to their strong safety and efficacy profile.
• Lentiviral vectors represent the fastest-growing virus type, supported by expanding CAR-T and cell therapy applications.
• Gene therapy is the leading application segment, accounting for nearly 47% of total market revenue in 2025.
• North America leads the market with approximately 54% share, driven by concentrated clinical trials and manufacturing investments.
• CDMOs are the fastest-growing end-user segment as outsourcing of viral vector production accelerates globally.
Overview of the Viral Vector Manufacturing Market
Viral vector manufacturing encompasses the development, production, purification, and quality control of viral delivery systems used in gene therapy, cell therapy, and vaccines. These processes require highly specialized infrastructure, advanced bioprocessing technologies, and strict adherence to current Good Manufacturing Practice (cGMP) regulations. As gene therapies increasingly target rare genetic disorders, oncology indications, and inherited diseases, viral vectors have become indispensable tools in modern medicine.
The market's growth is strongly linked to the commercialization of gene therapies that offer one-time, potentially curative treatments. Regulatory milestones achieved by therapies such as Zolgensma, Luxturna, and Hemgenix have validated the clinical and commercial viability of viral vector-based treatments. This validation has encouraged both public and private stakeholders to invest in large-scale manufacturing capabilities. Leading segments within the market include AAV-based manufacturing for in vivo gene therapy and lentiviral vector production for ex vivo cell therapies, while North America remains the dominant geographical region due to its innovation ecosystem and regulatory support.
Market Segmentation Analysis
The viral vector manufacturing market is segmented based on virus type, application, and end-user, reflecting the diversity of technologies and stakeholders involved in this rapidly evolving field. Virus type segmentation plays a critical role, as different vectors offer unique advantages depending on therapeutic requirements. AAV vectors dominate the market due to their non-integrating nature, low immunogenicity, and ability to target a wide range of tissues. These characteristics make them particularly suitable for in vivo gene therapy applications addressing rare genetic disorders and inherited diseases.
Lentiviral vectors, on the other hand, are gaining traction due to their ability to integrate into the host genome, which is essential for long-term gene expression in ex vivo cell therapies such as CAR-T and CAR-NK treatments.
Application-based segmentation highlights gene therapy as the leading segment, driven by the growing number of approved therapies and a strong clinical pipeline targeting oncology, rare diseases, and neurological disorders.
Vaccine development represents the fastest-growing application segment, supported by the validation of viral vector-based platforms during the COVID-19 pandemic and their subsequent adoption for infectious disease prevention and cancer immunotherapy. End-user segmentation reveals biotechnology and pharmaceutical companies as the largest contributors to market revenue, while CDMOs are experiencing the fastest growth as biopharma companies increasingly outsource complex manufacturing processes to specialized partners with scalable infrastructure and regulatory expertise.
Regional Insights into the Viral Vector Manufacturing Market
North America continues to lead the viral vector manufacturing market, supported by a mature biopharmaceutical ecosystem, extensive venture capital funding, and a high concentration of gene therapy clinical trials. The United States plays a central role, benefiting from progressive regulatory pathways such as the FDA's Regenerative Medicine Advanced Therapy designation, which accelerates clinical development and commercialization timelines. Canada is also strengthening its position through government-backed investments in biomanufacturing infrastructure and regulatory alignment with U.S. authorities, enhancing regional competitiveness.
Europe represents a significant share of the global market, driven by strong manufacturing capabilities in countries such as Germany, the United Kingdom, France, and Switzerland. Harmonized regulatory oversight under the European Medicines Agency facilitates cross-border clinical development and centralized marketing authorization, enabling efficient market access. However, pricing and reimbursement challenges persist, as health technology assessment bodies apply stringent cost-effectiveness criteria to high-cost gene therapies.
Asia Pacific is the fastest-growing regional market, fueled by supportive government policies, rising healthcare expenditure, and cost advantages in manufacturing. China leads regional growth through substantial investments in cell and gene therapy infrastructure and regulatory reforms that accelerate approval timelines. India is emerging as a strategic manufacturing hub, leveraging its skilled workforce, established biologics expertise, and government incentives to attract domestic and international investment in viral vector production.
Get Custom Insights Designed for Your Business: https://www.persistencemarketresearch.com/request-customization/26923
Market Drivers
The primary driver of the viral vector manufacturing market is the accelerating pace of gene therapy approvals and the evolution of reimbursement frameworks that support high-cost, one-time treatments. Regulatory initiatives such as orphan drug designations and expedited approval pathways have reduced development timelines, enabling faster commercialization and increasing demand for large-scale manufacturing. In parallel, value-based reimbursement models and installment payment schemes introduced in key markets are improving the economic feasibility of gene therapies, encouraging manufacturers to invest in expanded production capacity.
Market Restraints
Despite strong growth prospects, the viral vector manufacturing market faces significant restraints related to manufacturing complexity and regulatory compliance costs. Producing viral vectors under cGMP conditions requires sophisticated infrastructure, specialized expertise, and extensive quality control measures, all of which drive up production costs. Regulatory inconsistencies between major agencies, particularly regarding potency assays and release testing, further complicate global market access and increase development timelines, posing challenges for smaller biotech firms and academic institutions.
Market Opportunities
Emerging opportunities in the viral vector manufacturing market are closely linked to advancements in bioprocess optimization and platform technology standardization. Artificial intelligence and machine learning tools are increasingly being used to optimize upstream and downstream processes, improving yield predictability and reducing batch-to-batch variability. Standardized manufacturing platforms developed by leading technology providers are enabling faster process development and more efficient scale-up, particularly for CDMOs serving multiple clients. These innovations are expected to significantly reduce costs and enhance scalability across the market.
Checkout Now & Download Complete Market Report: https://www.persistencemarketresearch.com/checkout/26923
Company Insights
The viral vector manufacturing market is moderately consolidated, with a mix of vertically integrated pharmaceutical companies and specialized CDMOs competing for market share. Key players operating in the market include:
• Thermo Fisher Scientific Inc.
• Lonza Group AG
• Merck KGaA
• Catalent, Inc.
• Sartorius AG
• Oxford Biomedica plc
• FUJIFILM Diosynth Biotechnologies
• Charles River Laboratories International, Inc.
• WuXi Biologics (Cayman) Inc.
• AGC Biologics
• Novartis AG
• Takara Bio Inc.
• Aldevron, LLC
• Batavia Biosciences B.V.
• Resilience
Market Segmentation
By Virus Type
Adeno-Associated Virus (AAV) Vectors
Lentiviral Vectors
Adenoviral Vectors
Retroviral Vectors
Others
By Application
Gene Therapy
Vaccine Development
Cancer Therapy
Research & Development
Others
By End-user
Biotechnology Companies
Pharmaceutical Companies
Contract Development & Manufacturing Organizations (CDMOs)
Academic & Research Institutes
By Region
North America
Europe
East Asia
South Asia & Oceania
Latin America
Middle East & Africa
Recent developments highlight the market's dynamic nature. In June 2025, ProBio opened a flagship plasmid and viral vector manufacturing facility in Hopewell, New Jersey, strengthening U.S. capacity for advanced cell and gene therapy development. In September 2025, DINAMIQS inaugurated a state-of-the-art cGMP viral vector manufacturing facility in Zurich, Switzerland, featuring modular, single-use technologies designed to address scalability and compliance challenges.
Conclusion
The viral vector manufacturing market is entering a transformative phase, driven by the maturation of gene therapy, expanding cell therapy pipelines, and the validation of viral vector-based vaccine platforms. With strong growth projected through 2032, the market is characterized by rapid technological innovation, increasing outsourcing to CDMOs, and significant regional expansion, particularly in Asia Pacific. While manufacturing complexity and regulatory challenges remain, ongoing advancements in bioprocess optimization and standardization are expected to unlock new efficiencies and opportunities. As viral vectors continue to enable breakthrough therapies for previously untreatable diseases, their manufacturing ecosystem will remain a critical pillar of the global biopharmaceutical industry.
Read More Related Reports:
Life Sciences Aggregate-Spending Market https://www.persistencemarketresearch.com/market-research/life-sciences-aggregate-spending-sunshine-law-tracking-and-reporting-market.asp
GMP Cell Banking Service Market https://www.persistencemarketresearch.com/market-research/gmp-cell-banking-service-market.asp
Medical Marijuana Market https://www.persistencemarketresearch.com/market-research/medical-marijuana-market.asp
Urothelial Carcinoma Diagnostics Market https://www.persistencemarketresearch.com/market-research/urothelial-carcinoma-diagnostics-market.asp
Contact Us:
Persistence Market Research
Second Floor, 150 Fleet Street, London, EC4A 2DQ, United Kingdom
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Viral Vector Manufacturing Market Outlook 2025-2032: Strong CAGR of 23.5%, Says Persistence Market Research here
News-ID: 4335494 • Views: …
More Releases from Persistence Market Research
India Aluminum Beverage Can Market Size to Reach US$ 0.8 Bn by 2032 - Persistenc …
The India aluminum beverage can market is undergoing a significant transformation, driven by changing consumer lifestyles, rising urbanization, and a noticeable shift toward sustainable and convenient packaging formats. Aluminum beverage cans are increasingly preferred across carbonated soft drinks, energy drinks, sports beverages, alcoholic drinks, and ready-to-drink juices due to their lightweight structure, portability, fast chilling properties, and superior recyclability. In India, where on-the-go consumption is accelerating rapidly, aluminum cans are…
Medical Devices Packaging Market Size to Reach US$ 41.57 Billion by 2032 - Persi …
The medical devices packaging market plays a vital role within the global healthcare ecosystem, acting as a protective and regulatory bridge between manufacturers and end users. Medical device packaging refers to specialized materials and formats designed to safeguard medical instruments, implants, diagnostic tools, and consumables throughout storage, transportation, and clinical use. These packaging solutions are engineered to maintain sterility, prevent contamination, ensure ease of handling, and comply with strict regulatory…
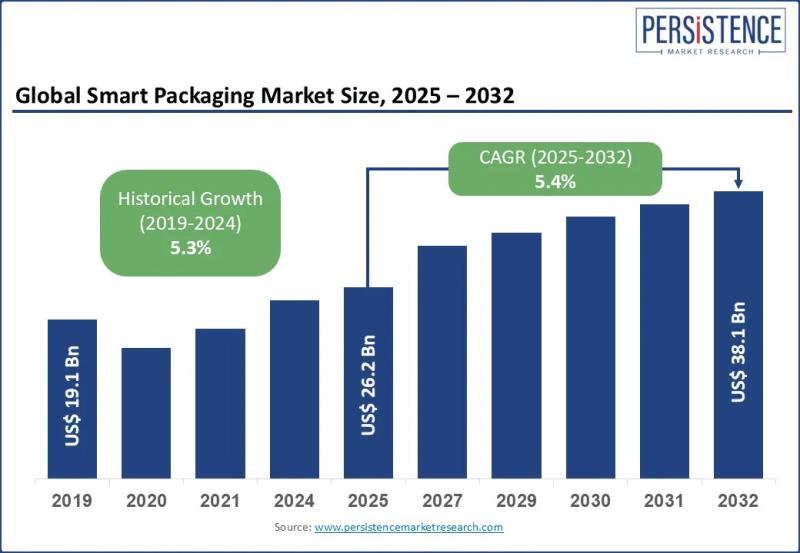
Smart Packaging Market Size Valued at US$ 26.2 Bn in 2025, Projected to Reach US …
The smart packaging market is rapidly transforming the global packaging landscape by integrating advanced technologies with traditional packaging materials to deliver enhanced functionality, traceability, and consumer engagement. Smart packaging refers to packaging systems embedded with features such as sensors indicators QR codes RFID tags and data tracking mechanisms that monitor product condition authenticity and movement across the supply chain. These solutions are increasingly adopted as businesses shift from passive containment…

Football Equipment Market Set for Strong Global Growth Through 2032
The global football equipment market continues to display resilient growth driven by rising participation in football across all age groups, expanding commercial opportunities, and technological advancements in sports gear. The industry is expected to grow from an estimated US$ 18.7 billion in 2025 to approximately US$ 24.1 billion by 2032, registering a compound annual growth rate (CAGR) of 3.7% over the forecast period.
➤ Download Your Free Sample & Explore Key…
More Releases for Vector
Growing Prevalence Of Vector-Borne Diseases Fuels Vector Control Market: Pivotal …
Stay ahead with our updated market reports featuring the latest on tariffs, trade flows, and supply chain transformations.
Vector Control Market Size Growth Forecast: What to Expect by 2025?
The market size of vector control has been experiencing robust growth in the past years, and it's predicted to increase from $20.53 billion in 2024 to $22.01 billion in 2025, with a compound annual growth rate (CAGR) of 7.2%. Factors such as urbanization…
Global Viral Vector Manufacturing Market Size by Application, Type, and Geograph …
USA, New Jersey- According to Market Research Intellect, the global Viral Vector Manufacturing market in the Internet, Communication and Technology category is projected to witness significant growth from 2025 to 2032. Market dynamics, technological advancements, and evolving consumer demand are expected to drive expansion during this period.
The viral vector manufacturing market is witnessing accelerated growth due to the rising demand for gene therapies and vaccines. These vectors are essential tools…
Prominent Viral Vector Manufacturing Market Trend for 2025: Product Innovation I …
How Are the key drivers contributing to the expansion of the viral vector manufacturing market?
The escalation in the occurrence of both cancer and contagious diseases is anticipated to spur the expansion of the viral vector manufacturing market in the future. An infectious disease is a condition caused by a virus or its harmful byproduct which spreads to a susceptible organism through contact with an infected individual, creature, or object. Cancer…
Emerging Lentiviral Vector Market Trend 2025-2034: Technological Advancements In …
How Is the Lentiviral Vector Market Projected to Grow, and What Is Its Market Size?
The lentiviral vector market will grow from $14.37 billion in 2024 to $16.62 billion in 2025, at a CAGR of 15.7%. This market is expanding due to the growing prevalence of genetic disorders, increasing demand for gene delivery systems, biotech and pharmaceutical investments, and regulatory approvals for related therapies.
The lentiviral vector market is expected to grow…
Viral Vector Manufacturing Market Report 2024 - Viral Vector Manufacturing Marke …
"The Business Research Company recently released a comprehensive report on the Global Viral Vector Manufacturing Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive…
Adeno-Associated Virus Vector Manufacturing Market - Building Better Therapies: …
Newark, New Castle, USA - Growth Plus Reports has published a new report on Adeno-Associated Virus Vector Manufacturing Market, which includes a detailed analysis based on competitors and important market segments (2023-2031). The Global Adeno-Associated Virus Vector Manufacturing provides a thorough analysis of many segments such as opportunities, market size, developments, innovation, sales, and overall growth of leading players. The research is based on primary and secondary statistical data, and…
