Press release
Rare Disease Therapeutics Market to Reach US$ 495.27 Billion by 2033 at 13.8% CAGR; North America Leads with 39% Share - Key Players: Roche, Novartis, Pfizer
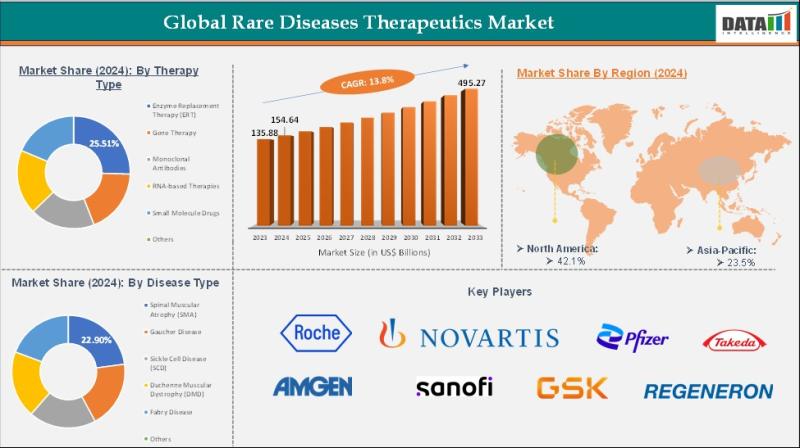
The Global Rare Disease Therapeutics Market reached US$ 135.88 billion in 2023 and increased to US$ 154.64 billion in 2024, and is expected to reach US$ 495.27 billion by 2033, growing at a CAGR of 13.8% during the forecast period 2025-2033. The market is witnessing robust expansion driven by the growing recognition of rare genetic and chronic disorders, increasing patient identification rates, and a strong shift toward precision and personalized medicine.Advancements in molecular diagnostics, next-generation sequencing, and genomics are enabling earlier and more accurate diagnosis of rare diseases, thereby accelerating demand for innovative therapeutic solutions such as enzyme replacement therapies, gene and cell therapies, monoclonal antibodies, and RNA-based drugs. Regulatory support through orphan drug designations, accelerated approval pathways, and financial incentives is further encouraging pharmaceutical and biotechnology companies to invest in rare disease drug development. North America is expected to maintain market leadership due to its advanced healthcare infrastructure, strong reimbursement frameworks, and high R&D investments, while emerging regions are demonstrating significant growth potential owing to rising disease awareness, improving healthcare access, and expanding diagnostic capabilities.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/rare-disease-therapeutics-market?sai-v
The Rare Disease Therapeutics Market encompasses the global development and commercialization of drugs and treatment solutions designed to diagnose, manage, and treat rare and orphan diseases.
Key Developments
✅ December 2025: Regulatory agencies globally released updated guidance frameworks to accelerate approvals for rare disease therapies, emphasizing adaptive trials, surrogate endpoints, and real-world evidence.
✅ October 2025: Biotech and pharmaceutical firms reported robust late-stage clinical progress for multiple rare disease assets, including gene therapies, RNA-based treatments, and enzyme replacement programs addressing high-unmet needs.
✅ August 2025: Major health systems expanded patient registry and genomic screening initiatives to improve diagnosis rates and support decentralized rare disease clinical research.
✅ June 2025: Payors and healthcare policymakers revised reimbursement models to facilitate broader patient access to high-cost rare disease therapeutics, including value-based agreements.
✅ April 2025: Research networks and international consortia launched collaborative platforms to aggregate multi-omics data and accelerate discovery across rare genetic and metabolic disorders.
✅ February 2025: New epidemiological studies highlighted rising global prevalence estimates for several rare diseases, underscoring the need for expanded therapeutic pipelines and accelerated drug development.
Mergers & Acquisitions
✅ November 2025: A leading global pharmaceutical company acquired a rare disease biotech with a diversified pipeline of gene and enzyme therapies to strengthen its rare disease portfolio.
✅ July 2025: A specialty pharma firm partnered with a clinical-stage rare disease biotech to co-develop next-generation oligonucleotide and cell therapy assets.
✅ May 2025: A healthcare investor consortium acquired a rare disease drug developer to accelerate late-stage development and commercialization of targeted therapies.
Key Players
F. Hoffmann-La Roche Ltd | Novartis AG | Pfizer Inc. | Takeda Pharmaceutical Company Limited | Amgen Inc. | Sanofi S.A. | GlaxoSmithKline plc | Regeneron Pharmaceuticals, Inc. | Biogen Inc. | Sarepta Therapeutics | Others
Key Highlights
F. Hoffmann-La Roche Ltd - Holds a 18.9% share, driven by its strong orphan drug portfolio, leadership in rare oncology and neurology therapies, and robust global commercialization capabilities.
Novartis AG - Holds a 16.7% share, supported by advanced gene and cell therapy platforms, multiple approved rare disease treatments, and sustained investment in innovation for ultra-rare conditions.
Pfizer Inc. - Holds a 12.4% share, leveraging its broad specialty medicine pipeline, global regulatory expertise, and strategic acquisitions expanding its rare disease footprint.
Takeda Pharmaceutical Company Limited - Holds a 11.1% share, driven by its strong focus on rare hematology, immunology, and genetic disorders, along with a well-established orphan drug development strategy.
Amgen Inc. - Holds a 9.6% share, benefiting from biologics expertise, precision medicine approaches, and continued expansion into rare inflammatory and metabolic diseases.
Sanofi S.A. - Holds a 8.5% share, supported by its leadership in rare genetic diseases, enzyme replacement therapies, and strong engagement with patient communities worldwide.
GlaxoSmithKline plc - Holds a 6.4% share, contributing through its immunology and rare infectious disease programs, along with selective investments in gene therapy research.
Regeneron Pharmaceuticals, Inc. - Holds a 5.7% share, recognized for its antibody discovery platforms, rapid translational research, and growing presence in rare inflammatory and genetic disorders.
Biogen Inc. - Holds a 4.9% share, focused on rare neurological and neuromuscular diseases, supported by deep CNS expertise and a diversified late-stage pipeline.
Sarepta Therapeutics - Holds a 3.8% share, specializing in rare neuromuscular disorders with a strong emphasis on Duchenne muscular dystrophy and next-generation gene therapies.
Others - Hold a 1.9% share, comprising emerging biotech firms, academic spin-offs, and niche developers targeting ultra-rare and underserved disease areas.
Purchase this report before year-end and unlock an exclusive 30% discount: https://www.datamintelligence.com/buy-now-page?report=rare-disease-therapeutics-market?sai-v
(Purchase 2 or more Reports and get 50% Discount)
Market Drivers
- Rising prevalence and improved diagnosis of rare diseases due to advanced genetic testing and increased awareness among healthcare professionals.
- Growing focus on orphan drug development incentivized by regulatory frameworks offering benefits such as market exclusivity, tax credits, and accelerated approvals.
- Advancements in precision medicine, gene therapies, and biologics tailored to specific rare disease pathways.
- Increasing investments and funding by pharmaceutical and biotechnology companies targeting underserved patient populations.
- Expansion of patient advocacy groups and global rare disease networks improving disease understanding and driving demand for effective treatments.
- Supportive government initiatives and policy frameworks facilitating research, approvals, and access to rare disease therapeutics.
- Growing adoption of digital health technologies, real-world evidence (RWE), and clinical trial innovations to streamline drug development.
- Rising healthcare expenditure and improved access to specialty care and treatment centers in emerging markets.
Industry Developments
- Launch of novel gene therapies, RNA-based therapeutics, and targeted biologics addressing specific rare disease mechanisms.
- Strategic collaborations between biotech firms, large pharmaceutical companies, and research institutions to accelerate R&D.
- Increased mergers, acquisitions, and licensing agreements to strengthen rare disease focused portfolios.
- Expansion of clinical trial networks and global patient registries to enhance trial enrollment and data collection.
- Regulatory approvals of breakthrough therapies and orphan-designated drugs across major markets.
- Integration of artificial intelligence and machine learning to identify new therapeutic targets and optimize drug discovery.
- Growth of personalized medicine approaches, including tailored treatment plans based on genetic and biomarker profiling.
- Emergence of patient-centric care models and support services improving therapy adherence and outcomes.
Regional Insights
North America - 39% share: "Driven by robust biotech and pharmaceutical innovation, strong regulatory incentives for orphan drugs, advanced healthcare infrastructure, and significant R&D investments."
Europe - 29% share: "Supported by established rare disease policies, growing clinical research activity, comprehensive patient registries, and increasing therapeutic approvals."
Asia Pacific - 24% share: "Fueled by expanding healthcare infrastructure, rising awareness of rare diseases, improving regulatory frameworks, and increasing investments in biotechnology."
Latin America - 5% share: "Boosted by improving healthcare access, gradual increase in rare disease diagnosis, and rising demand for specialized therapies."
Middle East & Africa - 3% share: "Driven by growing healthcare modernization efforts, expanding rare disease awareness, and increasing investments to improve access to advanced treatments."
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/rare-disease-therapeutics-market?sai-v
Key Segments
By Therapy Type
Enzyme Replacement Therapy (ERT) holds a significant share, driven by its long-standing clinical use in managing inherited metabolic and lysosomal storage disorders and its proven ability to alleviate disease symptoms. Gene therapy is emerging as a high-growth segment, supported by advances in viral vector technologies, one-time treatment potential, and increasing regulatory approvals. Monoclonal antibodies contribute steadily due to their targeted mechanisms and expanding applications in rare and genetic diseases. RNA-based therapies, including antisense oligonucleotides and RNA interference treatments, are witnessing rapid adoption, driven by precision medicine approaches and success in neuromuscular and genetic disorders. Small molecule drugs maintain a stable presence owing to oral availability, ease of administration, and supportive or disease-modifying roles. Other therapy types, including combination and supportive treatments, add to overall market growth through adjunct use.
By Disease Type
Spinal Muscular Atrophy (SMA) represents a major segment, driven by early diagnosis, strong clinical awareness, and the availability of advanced gene and RNA-based therapies. Gaucher disease holds a substantial share due to established ERT options and long-term treatment adoption. Sickle Cell Disease (SCD) is experiencing strong growth, supported by increasing focus on disease-modifying and gene-based therapies. Duchenne Muscular Dystrophy (DMD) contributes significantly, driven by ongoing development of exon-skipping, gene, and molecular-based treatments. Fabry disease maintains steady demand, supported by enzyme replacement and emerging gene therapies. Other rare diseases collectively contribute to market expansion as research pipelines broaden and diagnostic capabilities improve.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Rare Disease Therapeutics Market to Reach US$ 495.27 Billion by 2033 at 13.8% CAGR; North America Leads with 39% Share - Key Players: Roche, Novartis, Pfizer here
News-ID: 4332343 • Views: …
More Releases from DataM intelligence 4 Market Research LLP

Ginseng Dog Supplement Market Industry Analysis & Growth Outlook
Leander, Texas and Tokyo, Japan - Feb.04.2026
As per DataM intelligence research report "Global Ginseng Dog Supplement Market reached US$ 129.21 million in 2022 and is expected to reach US$ 209.07 million by 2031, growing with a CAGR of 6.2% during the forecast period 2024-2031."
The market is driven by rising pet humanization and demand for natural pet health supplements. Powder and chewable formats dominate adoption. Pet owners and veterinary channels are…
Artificial Intelligence Chip Market Ppt, Size, Share, Trends & Forecast 2030
Leander, Texas and Tokyo, Japan - Feb.04.2026
As per DataM intelligence research report "The global Artificial Intelligence (AI) Chip market was valued at US$ 25.12 billion in 2022 and is estimated to grow at a CAGR of 38.41% during the forecast period (2023-2030) to reach a value of US$ 335.02 billion in 2031."
Growth is fueled by rapid adoption of AI in data centers, edge computing, and consumer electronics. GPUs, ASICs, and…

Polyvinyl Alcohol Films Market Price, Size, Share, Trends & Forecast 2030
Leander, Texas and Tokyo, Japan - Feb.04.2026
As per DataM intelligence research report "Global Polyvinyl Alcohol (PVA) Films Market reached US$ 429.0 million in 2023 and is expected to reach US$ 720.7 billion by 2031, growing with a CAGR of 6.7% during the forecast period 2024-2031."
The market is driven by rising demand for water-soluble and biodegradable packaging solutions. Detergent packaging, agrochemical pouches, and embroidery applications dominate usage. Environmental regulations and shift…
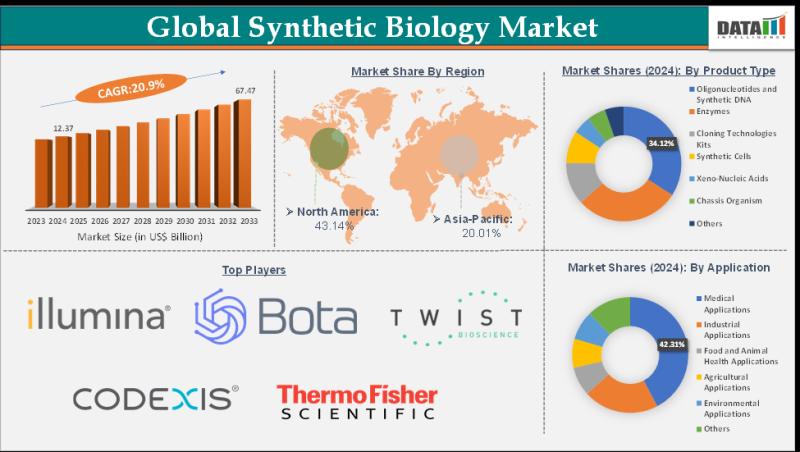
Synthetic Biology Market Set for Explosive Growth to USD 67.47 Billion by 2033, …
The Global Synthetic Biology Market size reached USD 12.37 Billion in 2024 from USD 10.38 Billion in 2023 and is expected to reach USD 67.47 Billion by 2033, growing at a CAGR of 20.9% during the forecast period 2025-2033.
Market growth is driven by expanding applications in healthcare, agriculture, and biofuels, alongside surging demand for sustainable biomanufacturing solutions. Advancements in gene editing tools like CRISPR, rising investments in bioeconomy initiatives, growing…
More Releases for Hold
Global Legal Hold Software Market Size by Application, Type, and Geography: Fore …
According to Market Research Intellect, the global Legal Hold Software market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The Legal Hold Software market is steadily expanding as organizations face increasing legal and regulatory scrutiny. This…
Twitch takes hold of the US Game Streaming Market can it hold its dominance? : K …
From the year 2011, when the game streaming platforms comes to a rise, Twitch was already a budding application, which started off slow but spread its wings in 2014, with celebrities coming to make a proper environment for budding players. By 2022, Twitch had almost 9 Million streamers, among which nearly everyone had a minimum 25 Million viewers, stating a 5% month-on-month (MOM) growth.
STORY OUTLINE
• Twitch TV has a greater number…
Developing Markets Hold Immense Potential for Telemedicine
Telemedicine and its applications have been growing rapidly, with technological advancements fuelling the sector’s growth. According to a new research report by RNCOS, the developing markets, such as India and China are largely underpenetrated for telemedicine, and hence, represent a major opportunity area. The telecommunications network and technologies are growing at an unprecedented rate in these economies, where the demographic profiles are large, and non-uniform healthcare facilities are available.…
Oswego BOA To Hold Public Meeting
Oswego, NY, September 20, 2011 – The Oswego Brownfield Opportunity Area (BOA) Steering Committee invites residents, business owners, property owners and others who have an interest in redevelopment of the waterfront, downtown and nearby industrial areas to a public meeting on Tuesday, September 27, 2011 at 7:00 p.m. at the Econo Lodge, 70 E. 1st Street. Meeting attendees will discuss options for redevelopment of key sites and areas within the…
SF SPCA to Hold Art Show Benefit
The San Francisco SPCA is pleased to announce its first art exhibition/benefit at the Leanne B. Roberts Animal Care Center Gallery, entitled “Characters, Friends and Neighbors.” This new endeavor will get art on the walls of the beautiful facility, feature a diverse group of local artists, and most importantly support the SF SPCA’s mission. 25% of all art sales from the exhibitions will go directly to the SF SPCA.
For…
Innermost Secrets Hold Educational Symposium
Cardiff, United Kingdom (12 November 2010) - Innermost Secrets, a leading fertility clinic, provide an educational symposium for patients and medical professionals.
Cardiff-based private fertility clinic, Innermost Secrets, was the first in the UK to launch a service for pregnant women at risk of premature birth and late miscarriage earlier in 2010. In 2011 they will be raising awareness of being ‘Born Too Soon’ with an educational symposium for patients and…