Press release
In-Silico Clinical Trials Market to Reach US$ 6.39 Billion by 2033 at 5.5% CAGR; North America Leads with 42% Share - Key Players: Certara, Dassault Systèmes, Simulations Plus
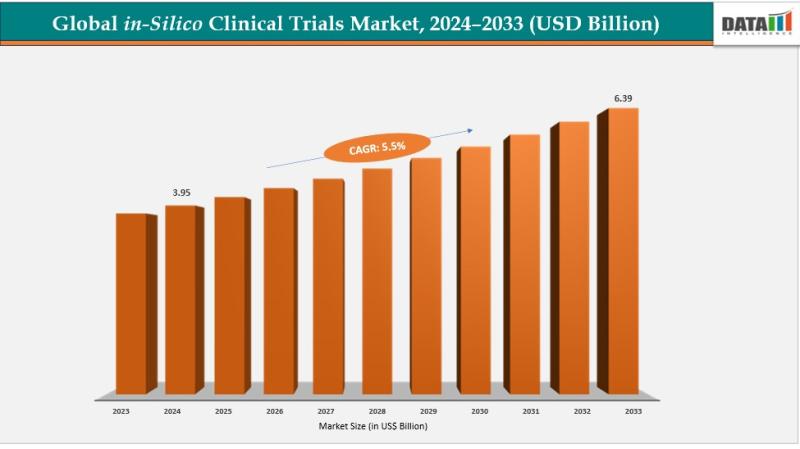
The In-Silico Clinical Trials Market reached US$ 3.76 billion in 2023 and increased to US$ 3.95 billion in 2024, and is expected to reach US$ 6.39 billion by 2033, growing at a CAGR of 5.5% during the forecast period 2025-2033. The market is experiencing steady growth driven by advancements in computational modeling, increasing adoption of artificial intelligence (AI) in drug development, and the need to reduce the high cost and long timelines associated with traditional clinical trials.In-silico clinical trials leverage advanced computing power, AI algorithms, and mathematical models to simulate biological, chemical, and physiological processes, enabling the prediction of drug safety, efficacy, and device performance. Expanded access to clinical and real-world data is improving model accuracy and reliability, accelerating decision-making across pharmaceutical and medical device development pipelines. Virtual trials allow pharmaceutical companies to significantly reduce development costs and time to market, while medtech companies benefit from simulating device performance before human testing. Contract research organizations (CROs) are increasingly offering in-silico services as value-added solutions, driving investments in specialized software platforms. Furthermore, gradual regulatory acceptance of model-informed evidence is strengthening the long-term adoption of in-silico clinical trials globally.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/in-silico-clinical-trials-market?sai-v
The In-Silico Clinical Trials Market refers to the market for computer-based simulation technologies and software used to model, predict, and analyze the safety and efficacy of medical treatments and interventions, reducing reliance on traditional human or animal trials.
Key Developments
✅ December 2025: Industry analyses highlighted strong global growth momentum for in-silico clinical trials, driven by increased adoption of AI-based modeling, virtual patient simulations, and integration of real-world evidence across drug and medical device development.
✅ November 2025: Market outlooks projected significant expansion of the in-silico clinical trials market through 2033, supported by digital validation approaches, model-informed drug development, and rising pharmaceutical and MedTech adoption.
✅ June 2025: In-silico platform providers enhanced cloud-based capabilities and advanced simulation tools to improve scalability and predictive accuracy in virtual trial environments.
✅ May 2025: Technology developers expanded computational modeling libraries and biological datasets to support more complex disease modeling and treatment response simulations.
✅ April 2025: Regulatory authorities, including major global agencies, reiterated support for alternative testing methodologies, encouraging the use of in-silico models alongside traditional preclinical and clinical evaluation pathways.
Mergers & Acquisitions
✅ October 2025: A global life sciences tools company acquired a clinical trial data and software solutions provider to strengthen digital trial execution, analytics, and simulation-enabled research capabilities.
✅ August 2025: A digital health technology firm partnered with a computational modeling company to integrate in-silico simulation tools into clinical development workflows.
✅ June 2025: A healthcare analytics company acquired a modeling and simulation startup to expand its presence in virtual trials and model-informed clinical decision-making.
Key Players
Certara | Dassault Systèmes | InSilicoTrials Technologies | Simulations Plus | VeriSIM Life | Physiomics Plc | ANSYS Inc. | Insilico Medicine | Others
Key Highlights
Certara - Holds a 22.4% share, driven by its strong leadership in biosimulation software, regulatory-grade modeling platforms, and widespread adoption across pharmaceutical and biotech companies for in-silico clinical trials.
Dassault Systèmes - Holds a 18.1% share, supported by its BIOVIA platform, advanced systems biology modeling, and deep integration of simulation technologies across drug discovery and development workflows.
Simulations Plus - Holds a 14.6% share, leveraging its expertise in pharmacokinetics/pharmacodynamics (PK/PD) modeling, physiologically based pharmacokinetic (PBPK) software, and strong acceptance by regulatory agencies.
InSilicoTrials Technologies - Holds a 11.3% share, driven by its cloud-based in-silico trial platforms, virtual patient modeling, and focus on reducing cost and timelines in clinical development.
VeriSIM Life - Holds a 9.5% share, recognized for its quantitative systems pharmacology (QSP) solutions, virtual organ simulations, and growing use in precision medicine and rare disease research.
Insilico Medicine - Holds a 8.2% share, benefiting from AI-driven drug discovery combined with simulation-based clinical trial optimization and strong investment in machine learning technologies.
Physiomics Plc - Holds a 6.1% share, focused on mathematical modeling, oncology-centric simulations, and collaborations with academic and pharmaceutical research institutions.
ANSYS Inc. - Holds a 5.0% share, contributing through advanced multiphysics simulation capabilities increasingly applied to biomedical modeling, medical device validation, and digital twin development.
Others - Hold a 4.8% share, comprising emerging technology providers, academic spin-offs, and niche software firms supporting specialized in-silico clinical trial applications.
Purchase this report before year-end and unlock an exclusive 30% discount: https://www.datamintelligence.com/buy-now-page?report=in-silico-clinical-trials-market?sai-v
(Purchase 2 or more Reports and get 50% Discount)
Market Drivers
- Increasing adoption of digital technologies and simulation tools to optimize clinical trial design and reduce time-to-market for new drugs and therapies.
- Rising pressure to cut high costs associated with traditional clinical trials and improve return on investment in pharmaceutical R&D.
- Growing emphasis on patient safety and the need to predict adverse events or efficacy outcomes prior to real-world testing.
- Advancements in computational biology, artificial intelligence (AI), machine learning (ML), and high-performance computing enabling robust simulation models.
- Supportive regulatory initiatives encouraging the use of in-silico methods for drug development and personalized medicine.
- Increasing demand for virtual patient modeling to enhance trial efficiency and reduce dependency on large patient cohorts.
- Expansion of personalized healthcare and precision medicine requiring sophisticated predictive analytics and simulation frameworks.
- Growing collaborations between pharmaceutical companies, technology providers, and research institutions to integrate in-silico approaches into clinical development.
Industry Developments
- Launch of AI-enabled in-silico clinical trial platforms with advanced predictive and modeling capabilities.
- Development of cloud-based simulation solutions to support scalable and collaborative trial simulations.
- Partnerships between tech companies and pharmaceutical firms to co-develop computational models for specific therapeutic areas.
- Acquisitions of specialized simulation software vendors to strengthen in-silico offerings among leading market players.
- Integration of real-world data (RWD) and electronic health records (EHR) into in-silico models to enhance accuracy and relevance.
- Expansion of regulatory frameworks and pilot programs by agencies to advance acceptance of virtual trial evidence.
- Increasing investments in R&D to improve digital twin technologies and patient-specific simulation tools.
- Emergence of customized simulation solutions for oncology, rare diseases, cardiovascular, and autoimmune therapeutic categories.
Regional Insights
North America - 42% share: "Driven by strong presence of pharmaceutical and biotechnology companies, advanced healthcare IT infrastructure, high R&D expenditure, and supportive regulatory landscape for digital trials."
Europe - 26% share: "Backed by increasing focus on digital health innovation, growing adoption of simulation tools in drug development, and collaborations between research institutions and industry."
Asia Pacific - 24% share: "Fueled by expanding pharmaceutical R&D activities, rising investment in healthcare technology, improving regulatory support for digital methodologies, and increasing clinical trial outsourcing."
Latin America - 5% share: "Boosted by improving healthcare infrastructure, rising awareness of digital trial benefits, and gradual adoption of simulation-based drug development tools."
Middle East & Africa - 3% share: "Driven by growing investment in healthcare modernization, adoption of digital health platforms, and emerging interest in advanced clinical trial approaches."
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/in-silico-clinical-trials-market?sai-v
Key Segments
By Application
Drug development holds a significant share, driven by the growing use of advanced modeling and simulation approaches to evaluate efficacy, safety, and dosing strategies while reducing time and cost in preclinical and clinical phases. Medical device evaluation is witnessing steady growth as companies increasingly rely on virtual testing to assess performance, usability, and safety across diverse patient populations. Regulatory submissions are gaining importance, supported by rising acceptance of digital evidence and simulation data by regulatory authorities to complement traditional clinical trial data. Post-market surveillance is expanding due to the need for continuous monitoring of product performance, safety, and real-world outcomes throughout the product lifecycle. Other applications, including protocol optimization and patient stratification, contribute to overall market growth through specialized use cases.
By End User
Pharmaceutical and biotech companies dominate the segment, driven by strong investments in R&D and the need to streamline development timelines while minimizing clinical risk. Medical device manufacturers represent a growing share, supported by increasing regulatory scrutiny and demand for efficient product validation methods. Academic and research institutes contribute significantly through innovation, validation studies, and collaborative research initiatives. Contract research organizations are witnessing rapid growth as sponsors increasingly outsource simulation, modeling, and analytical services to specialized partners. Other end users, including regulatory bodies and healthcare organizations, support market expansion through adoption of advanced evaluation and monitoring solutions.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release In-Silico Clinical Trials Market to Reach US$ 6.39 Billion by 2033 at 5.5% CAGR; North America Leads with 42% Share - Key Players: Certara, Dassault Systèmes, Simulations Plus here
News-ID: 4332249 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
Medical Transcription Service Market to Reach US$ 65.70 Billion by 2031 at 5.3% …
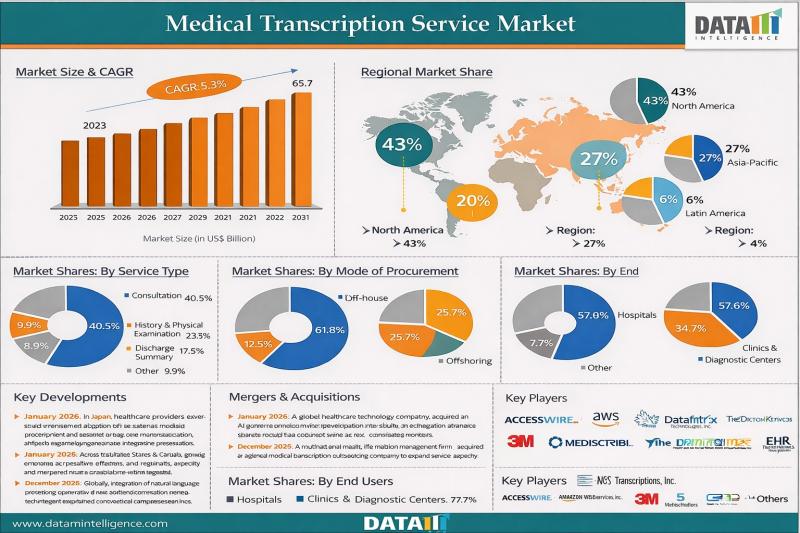
Medical Transcription Service Market reached US$ 43.46 billion in 2023 and is expected to reach US$ 65.70 billion by 2031, growing at a CAGR of 5.3% during the forecast period 2024-2031.
Medical transcription (MT) involves the accurate conversion of diverse healthcare audio recordings into structured written documentation, including clinical reports, consultation notes, discharge summaries, psychiatric evaluations, referrals, interviews, and patient medical histories dictated by healthcare professionals. These transcription outputs form a…
Urban Air Mobility Market Set for Explosive Growth to USD 54.03 Billion by 2032, …
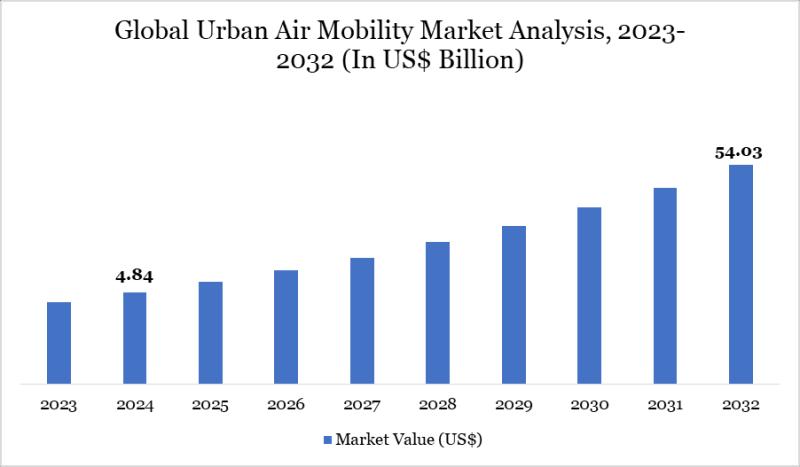
The Global Urban Air Mobility Market reached USD 4.84 billion in 2024 and is expected to reach USD 54.03 billion by 2032, growing with a robust CAGR of 35.20% during the forecast period 2025-2032.
Market growth is driven by rapid urbanization, surging demand for efficient short-distance transportation, and advancements in electric vertical takeoff and landing (eVTOL) aircraft. Supportive government regulations, substantial investments from aerospace giants like Joby Aviation and Lilium, expanding…
Stair Lifts Market to Reach USD 3.24 Billion by 2032 at 8.6% CAGR | North Americ …
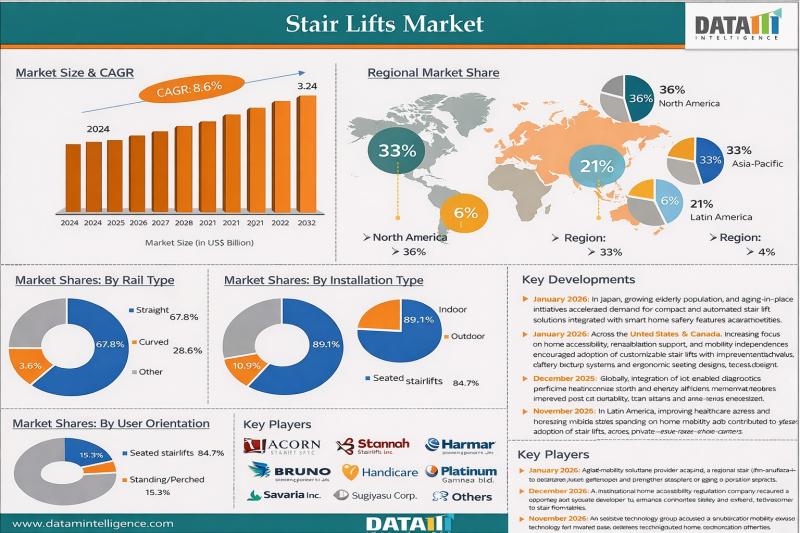
Stair Lifts Market reached USD 1.36 billion in 2024 and is expected to reach USD 3.24 billion by 2032, growing at a CAGR of 8.6% during the forecast period 2025-2032.
The stair lifts market is being driven by the rapidly ageing global population, increasing prevalence of mobility-related disabilities, and a growing preference for ageing-in-place solutions that enhance independence and quality of life for seniors and individuals with physical limitations. Stair lifts…
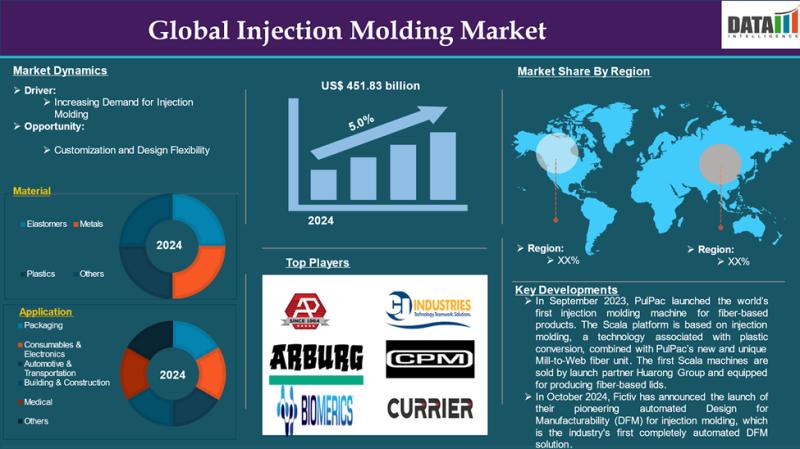
United States Injection Molding Market Insights 2033 | Growth in Key Industries, …
Injection Molding Market reached US$ 295 billion in 2024 and is expected to reach US$ 451.83 billion by 2033, growing at a CAGR of 5% during the forecast period 2025-2033
Get a Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/injection-molding-market?kb
Injection Molding Market: Trends, Services, and Innovations Shaping the Industry
The Injection Molding Market continues to evolve rapidly, driven by advancements in materials, technologies, and customized manufacturing solutions.…
More Releases for Hold
Global Legal Hold Software Market Size by Application, Type, and Geography: Fore …
According to Market Research Intellect, the global Legal Hold Software market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The Legal Hold Software market is steadily expanding as organizations face increasing legal and regulatory scrutiny. This…
Twitch takes hold of the US Game Streaming Market can it hold its dominance? : K …
From the year 2011, when the game streaming platforms comes to a rise, Twitch was already a budding application, which started off slow but spread its wings in 2014, with celebrities coming to make a proper environment for budding players. By 2022, Twitch had almost 9 Million streamers, among which nearly everyone had a minimum 25 Million viewers, stating a 5% month-on-month (MOM) growth.
STORY OUTLINE
• Twitch TV has a greater number…
Developing Markets Hold Immense Potential for Telemedicine
Telemedicine and its applications have been growing rapidly, with technological advancements fuelling the sector’s growth. According to a new research report by RNCOS, the developing markets, such as India and China are largely underpenetrated for telemedicine, and hence, represent a major opportunity area. The telecommunications network and technologies are growing at an unprecedented rate in these economies, where the demographic profiles are large, and non-uniform healthcare facilities are available.…
Oswego BOA To Hold Public Meeting
Oswego, NY, September 20, 2011 – The Oswego Brownfield Opportunity Area (BOA) Steering Committee invites residents, business owners, property owners and others who have an interest in redevelopment of the waterfront, downtown and nearby industrial areas to a public meeting on Tuesday, September 27, 2011 at 7:00 p.m. at the Econo Lodge, 70 E. 1st Street. Meeting attendees will discuss options for redevelopment of key sites and areas within the…
SF SPCA to Hold Art Show Benefit
The San Francisco SPCA is pleased to announce its first art exhibition/benefit at the Leanne B. Roberts Animal Care Center Gallery, entitled “Characters, Friends and Neighbors.” This new endeavor will get art on the walls of the beautiful facility, feature a diverse group of local artists, and most importantly support the SF SPCA’s mission. 25% of all art sales from the exhibitions will go directly to the SF SPCA.
For…
Innermost Secrets Hold Educational Symposium
Cardiff, United Kingdom (12 November 2010) - Innermost Secrets, a leading fertility clinic, provide an educational symposium for patients and medical professionals.
Cardiff-based private fertility clinic, Innermost Secrets, was the first in the UK to launch a service for pregnant women at risk of premature birth and late miscarriage earlier in 2010. In 2011 they will be raising awareness of being ‘Born Too Soon’ with an educational symposium for patients and…