Press release
United States Rare Neurological Disease Drugs Market to Reach US$ 269.7 Billion by 2031 | CAGR of 8.6% | Biologics & Orphan Drugs Drive Growth | Key Players: Novartis, Pfizer, Johnson & Johnson, US WorldMeds, Aquestive Therapeutics, Merz Pharma, Kedrion B
Rare Neurological Disease Drugs MarketThe Global Rare Neurological Disease Drugs Market reached US$ 139.4 billion in 2023 and is projected to grow to US$ 269.7 billion by 2031, expanding at a CAGR of 8.6% during the forecast period 2024-2031. Rare neurological diseases, also known as orphan neurological disorders, encompass a wide range of conditions affecting the central and peripheral nervous systems as well as the muscles. These disorders are classified as "rare" due to their low prevalence, typically affecting less than 1 in 2,000 people.
Examples of rare neurological disorders include Aicardi syndrome, Aicardi-Goutieres syndrome, Reflex Sympathetic Dystrophy Syndrome, Battaglia-Neri syndrome, Creutzfeldt Jakob Disease, and Agnosia, among others. These conditions often impact the brain and spinal cord, leading to diverse symptoms and challenges in diagnosis and treatment. Therapeutic approaches for rare neurological diseases include cognitive behavioral therapy, interpersonal psychotherapy, and cyberknife treatment. Additionally, pharmaceutical interventions commonly involve drugs such as levetiracetam (Keppra), topiramate (Topamax), lamotrigine (Lamictal), oxcarbazepine (Trileptal), and divalproex sodium (Depakote). These treatments aim to manage symptoms, reduce disease progression, and improve patient quality of life.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/rare-neurological-disease-drugs-market?Juli
Recent Developments:
✅ October 2025: A leading biopharmaceutical company announced FDA approval of a novel gene therapy for a specific rare neurological disorder, representing one of the first targeted therapeutic options for that condition and paving the way for additional orphan drug developments.
✅ August 2025: A global pharma firm expanded its portfolio by launching a next‐generation small molecule therapy designed to better cross the blood‐brain barrier, improving efficacy in treating certain rare neurodegenerative conditions.
✅ June 2025: Several major drug manufacturers initiated large‐scale, multi‐center clinical trials for new monoclonal antibody therapies aimed at slowing disease progression in rare neurological conditions with immune‐mediated mechanisms.
✅ April 2025: A biotechnology company secured orphan drug designation for its investigational antisense oligonucleotide therapy targeting a rare hereditary neurological disease, enabling regulatory incentives and accelerated development pathways.
✅ February 2025: A partnership was announced between a neuropharmaceutical developer and a research consortium to establish a global patient registry and biobank, supporting improved disease tracking, natural history studies, and faster recruitment for clinical trials.
Mergers & Acquisitions:
✅ November 2025: A major global biopharmaceutical company acquired a rare disease‐focused biotech firm to strengthen its neurological disorder pipeline with multiple orphan drug candidates in late‐stage development.
✅ September 2025: A large specialty pharma group completed the acquisition of a neurotherapeutics startup developing next‐generation small molecule and biologic therapies for rare neurological conditions, expanding its presence in central nervous system (CNS) disorders.
✅ July 2025: A global rare diseases consortium merged with a clinical development organization specializing in orphan drug regulatory strategy and trial execution, enhancing commercialization pathways for rare neurological therapies.
✅ May 2025: A leading neuroscience drug developer acquired a gene therapy platform provider to accelerate development of targeted gene‐based treatments for inherited neurological disorders.
✅ March 2025: A multinational pharmaceutical company entered a strategic acquisition of a neurogenetics research company to bolster its discovery and translational research capabilities in rare neurological disease mechanisms.
Buy Now & Unlock 360° Market Intelligence:-https://www.datamintelligence.com/buy-now-page?report=rare-neurological-disease-drugs-market?Juli
Key Players:
• Novartis AG - Holds 14.2% share, driven by its portfolio of innovative therapies targeting rare neurological disorders and strong global distribution networks.
• Pfizer, Inc. - Holds 12.8% share, fueled by its research in orphan CNS therapies and strategic partnerships for rare disease drug development.
• Johnson & Johnson Services, Inc. - Holds 11.5% share, supported by its neurotherapeutics pipeline and established commercialization capabilities.
• US WorldMeds LLC (Solstice Neurosciences LLC) - Holds 9.7% share, focusing on niche rare neurological disease treatments with targeted marketing strategies.
• Aquestive Therapeutics Inc. - Holds 8.3% share, driven by its specialized drug delivery platforms for CNS and rare disease drugs.
• Sanofi S.A. - Holds 7.9% share, supported by its extensive rare disease portfolio and collaborations in gene therapy development.
• Merck & Co., Inc. - Holds 7.2% share, leveraging its R&D pipeline in orphan neurological disorders and strategic acquisitions.
• CSL Ltd - Holds 6.5% share, focusing on rare disease biologics, including plasma-derived therapies for neurological conditions.
• Merz Pharma GmbH & Co. KGaA - Holds 5.8% share, backed by its specialty neurology and aesthetic neurology drug lines.
• Kedrion Biopharma Inc. - Holds 5.1% share, emphasizing rare neurological disease therapies and immunoglobulin-based treatments.
Market Segmentation:
➥ By drug type, biologics dominate with a 58% share due to their effectiveness in treating complex rare neurological disorders, while small molecules hold 42%, driven by oral formulations and lower production costs. By route of administration, intravenous (IV) therapies account for 54% of the market, preferred for biologics and hospital-based treatments, whereas oral formulations represent 46%, providing convenience for chronic therapy and at-home use.
➥ By application, Dravet syndrome holds a 16% share, supported by specialized antiepileptic treatments. Adrenoleukodystrophy accounts for 12%, driven by gene therapy and orphan drug adoption, while narcolepsy represents 10%, fueled by increasing awareness and new therapies. Angelman syndrome captures 8% of the market with targeted therapies in development, and amyotrophic lateral sclerosis (ALS) holds 14%, supported by disease-modifying treatments and clinical trials. Other rare neurological conditions make up the remaining 40%, reflecting the wide range of specialized care needs.
➥ By distribution channel, hospital pharmacies dominate with 52%, primarily due to intravenous biologics and specialized treatments. Retail pharmacies account for 30%, catering mainly to oral medications and follow-up prescriptions, while online pharmacies represent 18%, benefiting from convenience, telemedicine integration, and home delivery services. This segmentation highlights how drug types, administration routes, therapeutic applications, and distribution channels collectively shape the growth and accessibility of rare neurological disease drugs worldwide.
Speak to Our Analyst and Get Customization in the report as per your requirements:-https://www.datamintelligence.com/customize/rare-neurological-disease-drugs-market?Juli
Regional Insights:
North America dominates the market, holding approximately 38% share, driven by advanced healthcare systems, strong R&D investments, high patient awareness, and favorable reimbursement policies. The Europe region accounts for around 28%, supported by well-established healthcare networks, government initiatives for rare disease management, and orphan drug incentives.
Asia-Pacific is emerging as a high-growth region with a 20% share, fueled by increasing healthcare expenditure, expanding access to specialized care, rising prevalence of neurological disorders, and growing adoption of orphan drugs in countries such as Japan, China, and India.
Latin America and Middle East & Africa collectively hold about 14% of the market, constrained by limited healthcare infrastructure, low awareness of rare neurological disorders, and restricted access to advanced therapies, though improving government initiatives and private sector investments are expected to gradually boost growth in these regions.
Market Dynamics:
Drivers: Increasing Prevalence of Rare Neurological Diseases
The global rare neurological disease drugs market is primarily driven by the rising prevalence of rare neurological disorders. According to the World Health Organization (WHO), over 1 in 3 people were affected by neurological conditions as of May 2024, making these diseases a leading cause of illness and disability worldwide. Rare neurological diseases such as amyotrophic lateral sclerosis (ALS), Duchenne muscular dystrophy, vertical gaze palsy, and Huntington's disease are contributing to the growing demand for effective therapies. As reported by Orphanet in November 2023, the prevalence of chronic inflammatory demyelinating polyneuropathy is 3.7 per 100,000 individuals in Europe, ALS prevalence is 3.85 per 100,000 globally and 5.2 in the EU, and Huntington's disease affects 12 per 100,000 in the EU.
Additionally, strategic initiatives by key market players are accelerating growth. Partnerships, collaborations, clinical trials, and product approvals are expanding therapeutic options. For example, in July 2023, Biogen Inc. announced the acquisition of Reata Pharmaceuticals, Inc. for approximately $7.3 billion to strengthen its rare disease portfolio. In April 2023, Orphalan SA launched Cuvrior in the U.S. for adult patients with stable Wilson disease, while Biogen Inc. received FDA approval for QALSODY (tofersen) for ALS patients with SOD1 gene mutations, reflecting an increasing availability of targeted treatments.
Restraints
The market faces several challenges that may hinder growth, including the high cost of therapies, stringent regulatory requirements, limited awareness and diagnosis, and the small patient population typical of rare neurological diseases. Additional barriers include reimbursement challenges, limited treatment options, and a shortage of skilled and trained healthcare professionals, particularly in emerging markets. These factors collectively restrict widespread adoption despite the increasing prevalence of rare neurological disorders.
Key Developments:
May 2024: Biogen Inc announced that the European Commission (EC) granted marketing authorization under exceptional circumstances and maintained orphan designation for QALSODY (tofersen) for treating adults with amyotrophic lateral sclerosis (ALS) associated with a mutation in the superoxide dismutase 1 gene (SOD1-ALS).
April 2024: NS Pharma announced a research alliance with MiNA Therapeutics to develop therapies for rare diseases of the central nervous system.
February 2024: Roche Pharma India launched its breakthrough drug, Ocrevus (Ocrelizumab), for the treatment of multiple sclerosis (MS), expanding its neurology portfolio to address unmet patient needs in India.
September 2023: Harmony Biosciences Holdings, Inc. announced that the U.S. Food and Drug Administration (FDA) granted Orphan Drug designation to pitolisantf for the treatment of idiopathic hypersomnia (IH).
📌 Request for 2 Days FREE Trial Access: https://www.datamintelligence.com/reports-subscription
☛ Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
☛ Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg?Juli
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release United States Rare Neurological Disease Drugs Market to Reach US$ 269.7 Billion by 2031 | CAGR of 8.6% | Biologics & Orphan Drugs Drive Growth | Key Players: Novartis, Pfizer, Johnson & Johnson, US WorldMeds, Aquestive Therapeutics, Merz Pharma, Kedrion B here
News-ID: 4330667 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
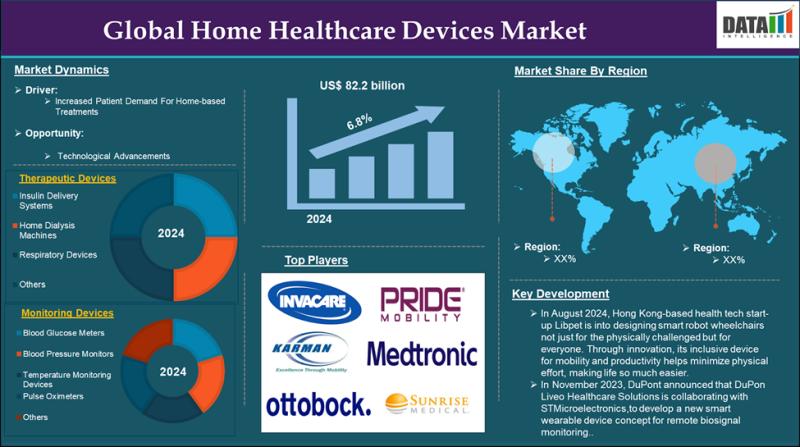
United States Home Healthcare Devices Market 2033 | Growth Drivers, Key Players …
Market Size and Growth
The Global Home Healthcare Devices Market reached US$46.25 billion in 2024 and is expected to reach US$82.27 billion by 2033, growing at a CAGR of 6.8% during the forecast period 2025-2033, according to DataM Intelligence report.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/home-healthcare-devices-market?sb
Key Development:
✅ In January 2026, Aveanna Healthcare announced plans to pursue multiple home health company acquisitions across…
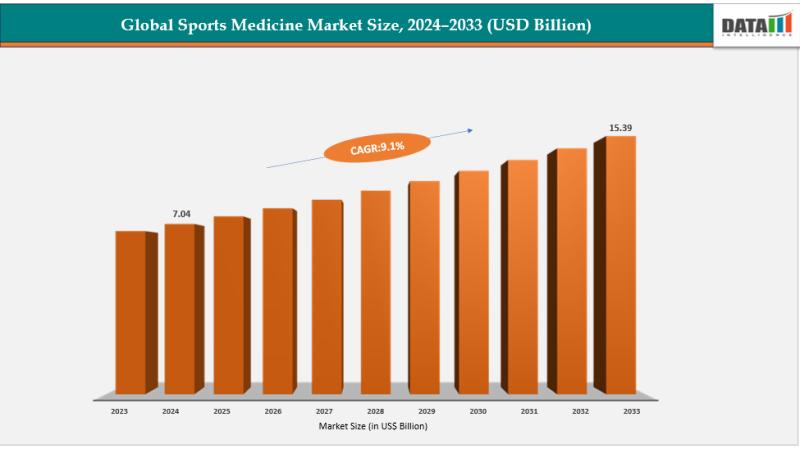
United States Sports Medicine Market 2033 | Growth Drivers, Key Players & Invest …
Market Size and Growth
The global sports medicine market size reached US$ 7.04 billion in 2024 is expected to reach US$ 15.39 billion by 2033, growing at a CAGR of 9.1% during the forecast period 2025-2033.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/sports-medicine-market?sb
Key Development:
United States: Recent Industry Developments
✅ In January 2026, Bioretec Oy was granted FDA Breakthrough Device Designation for its RemeOsTM DrillPin biodegradable…

Ovulation Disorder Diagnosis Market to Reach US$ 768.99 Million by 2031 at 5.0% …
Market Overview
The Global Ovulation Disorder Diagnosis Market was valued at US$ 522.60 million in 2023 and is projected to reach US$ 768.99 million by 2031, growing at a CAGR of 5.0% during the forecast period (2024-2031). Ovulation disorders represent one of the leading causes of infertility in women, accounting for a significant proportion of reproductive health challenges worldwide. These disorders involve irregularities or failure in egg production during the menstrual…
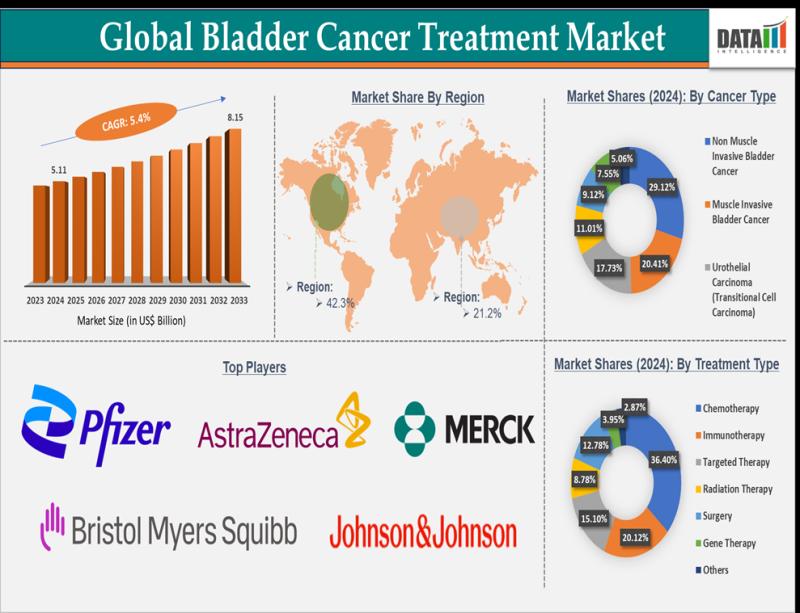
Bladder Cancer Treatment Market Size & Forecast 2033 | Expected to Reach US$ 8.1 …
Leander, Texas and Tokyo, Japan - Jan.30.2026
The global bladder cancer treatment market size reached US$ 5.11 Billion in 2024 and is expected to reach US$ 8.15 Billion by 2033, growing at a CAGR of 5.4% during the forecast period 2025-2033.
The bladder cancer treatment market is expanding as advances in targeted therapies, immunotherapy, and minimally invasive procedures improve patient outcomes. Treatments include surgery, intravesical therapy, chemotherapy, checkpoint inhibitors, and emerging antibody-drug…
More Releases for Holds
Mobile Robotics Market: What the Future Holds?
Allied Market Research published a new report, titled, "Mobile Robotics Market by Product (UGV, UAV, and AUV), Component (Hardware, Software, and Support & Services), Application (Logistics & Warehousing, Military & Defense, Healthcare, Domestics, Entertainment, Education, Agriculture & Forestry, and Others): Global Opportunity Analysis and Industry Forecast, 2021-2027."
The research report offers an in-depth analysis of the current market scenario, estimates, revolving aspects, and dynamic forces of the industry from 2021 to…
IFX ENTERPRISES HOLDS STRATEGIC FRANCHISE SUMMIT
San Diego, CA (November 3, 2011) – The leading strategic consulting firm for franchise organizations and suppliers servicing the franchise channel, IFX Enterprises will hold a Strategic Franchise Summit, November 30 – December 2, 2011. IFX and its CEO, Dan Martin, CFE, will provide strategies on how to maximize franchise development and operations learned over 20 years of experience.
IFX’s Strategic Franchise Summit will offer franchise organizations and executives…
IFX Holds First Franchise Supplier Executive Summit
San Diego Based Consulting Firm to Offer Strategies of Breaking into the Franchise Industry
SAN DIEGO, CA (August 2, 2011) – The leading applications service provider and strategic consulting firm for franchise organizations and suppliers servicing the franchise channel, IFX will hold its first Franchise Supplier Executive Summit, August 11-13, 2011 in San Diego, CA. The conference is designed for companies not currently marketing to the franchise channel, as well…
Avnet holds “ISS Academy” in Saudi Arabia
Avnet Technology Solutions, the leading Value added Distributor in the Middle East and Africa region recently held a special training program called “ISS Academy” in association with HP and Microsoft to educate its Preferred Partners, System Integrators and ISV’s on the latest news and updates from HP ISS world.
With leading the Market share over 50% HP Industry Standard Servers (ISS) finds its presence in almost every Data Center across…
Business Coaching Client Holds Grand Opening
Branford, CT; Denver, CO -- The Von Gehr Consulting Group, LLC is excited to announce that its client Rocky Mountain Roots Acupuncture & Herbal Medicine, LLC, will be holding an Open House of their new facilities. After engaging the Von Gehr Consulting Group's business coaching, business planning, and business consulting services in December of 2009, Rocky Mountain Roots (RMR) is organized and filled with excitement about their Open House.
"Rocky…
ADAOX Holds Partner Conference in Dubai
Dubai, United Arab Emirates, February 24, 2009: ADAOX Middle East, the regional business development centre of ESET NOD32 Antivirus, today announced that they held special partner conference and training session exclusively for their channel partners at the Tower Rotana in Dubai last week. The training session involved a demonstration on ESET products version 4 followed by technical training on ESET Enterprise and Mail Server Solution deployment and trouble shooting. Security…
