Press release
Aseptic Transfer System Market to Reach USD 3.0 Billion by 2035, Driven by Rising Sterile Biologics Production and Stringent Contamination-Control Regulations | TMR
The global Aseptic Transfer System Market was valued at US$ 1.5 billion in 2024 and is projected to reach more than US$ 3.0 billion by 2035, expanding at a compound annual growth rate (CAGR) of 6.7% from 2025 to 2035. Market growth is primarily driven by the rising production of sterile drugs, biologics, and vaccines, alongside increasingly stringent contamination-control and regulatory compliance requirements across pharmaceutical and biotechnology manufacturing facilities.Access key findings and insights from our Report in this sample -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=74065
The growing emphasis on contamination-free processing, product integrity, and patient safety has positioned aseptic transfer systems as a critical component of modern sterile manufacturing environments.
Market Overview
The aseptic transfer system (ATS) market plays a vital role in enabling contamination-free material transfer across pharmaceutical and biotechnology production environments. ATS solutions are designed to facilitate the safe transfer of powders, liquids, equipment, and components between controlled areas without compromising sterility.
As pharmaceutical pipelines increasingly focus on injectable drugs, biologics, vaccines, and advanced therapies, the need for reliable, validated, and compliant transfer systems has intensified. Traditional open-transfer processes present high contamination risks and regulatory challenges, prompting manufacturers to adopt closed and automated aseptic transfer solutions.
Modern ATS technologies incorporate closed containers, sterilizable connectors, isolators, gloveboxes, and transfer ports to maintain sterile conditions throughout the production cycle. These systems are now considered a best practice across sterile manufacturing operations, enabling improved efficiency, reduced human intervention, and enhanced compliance with Good Manufacturing Practices (GMP).
Key Market Growth Drivers
Rising Production of Sterile Drugs and Biologics
The increasing production of sterile drugs and biologics is one of the primary drivers of growth in the aseptic transfer system market. The pharmaceutical and biotechnology industries are experiencing sustained growth in demand for injectable drugs, monoclonal antibodies, vaccines, and other biologics that require highly controlled, sterile environments.
These products are extremely sensitive to microbial, particulate, and environmental contamination. Any compromise in sterility during material transfer can result in batch failures, costly recalls, and patient safety risks. Aseptic transfer systems ensure secure and sterile handling of materials throughout the manufacturing process, protecting product integrity and quality.
The rapid rise of advanced therapies, such as cell and gene therapies, further amplifies the need for precise and contamination-free transfer solutions. These therapies often involve small-batch production, high-value materials, and complex workflows, making closed and automated ATS solutions indispensable.
Manufacturers are increasingly adopting single-use, automated, and modular ATS technologies to minimize human intervention, reduce contamination risks, and improve operational efficiency. These systems not only enhance sterility assurance but also support scalability and flexibility across different manufacturing volumes.
Stringent Regulatory Requirements Driving Market Expansion
Stringent regulatory requirements represent a major growth driver for the aseptic transfer system market. Regulatory authorities such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other global health agencies enforce rigorous standards governing aseptic processing, contamination control, and environmental monitoring.
Compliance with these regulations is critical for pharmaceutical and biotechnology manufacturers. ATS solutions enable companies to safely transfer materials between controlled environments while preventing microbial, particulate, and cross-contamination-helping manufacturers meet regulatory expectations with greater confidence.
The rising demand for injectable drugs, biologics, and advanced therapy medicinal products has further increased regulatory scrutiny. Closed, automated, and single-use ATS technologies significantly reduce human contact, which remains one of the leading sources of contamination risk.
In addition to ensuring sterility, ATS solutions streamline validation, monitoring, and audit processes. Their ability to support consistent, repeatable, and well-documented transfer processes makes them essential tools for regulatory compliance as global standards continue to tighten.
Market Challenges and Barriers
Despite strong growth prospects, the aseptic transfer system market faces several challenges. High capital investment requirements, complex validation procedures, and the need for specialized technical expertise can limit adoption, particularly among smaller manufacturers.
Operational costs associated with installation, maintenance, and system qualification remain significant barriers. Additionally, ATS systems require skilled personnel to operate, validate, and maintain them, creating workforce-related challenges in some regions.
Nevertheless, ongoing technological advancements-such as modular designs, automation, and single-use systems-are gradually reducing these barriers and expanding adoption across a wider range of manufacturers.
Liquid Transfer System Segment Driving Market Growth
Aseptic Transfer System Market by System Type
The liquid transfer system segment accounted for the largest market share, holding 61.4% of total revenue in 2024. This dominance reflects the high volume of sterile liquid transfers required in pharmaceutical and biotechnology manufacturing.
Sterile liquids such as injectable drugs, vaccines, and biologics must be transferred between production stages under strict aseptic conditions. Liquid transfer ATS solutions are specifically designed to preserve sterility while minimizing the risk of microbial, particulate, and cross-contamination.
Technological innovations in liquid transfer systems-including single-use tubing, closed-system connectors, automated pumps, and real-time monitoring technologies-have significantly improved operational efficiency and sterility assurance. These systems reduce operator involvement, support GMP compliance, and minimize the risk of costly production disruptions.
Liquid transfer ATS solutions are highly versatile and scalable, making them suitable for both small-batch manufacturing and large-scale commercial production. Their adaptability has driven widespread adoption across pharmaceutical manufacturers and contract development and manufacturing organizations (CDMOs).
Regional Outlook
North America Leads the Global Market
North America dominated the global aseptic transfer system market in 2024, accounting for approximately 35.8% of total revenue. The region's leadership is supported by a well-established pharmaceutical manufacturing infrastructure, advanced biotechnology capabilities, and a highly regulated operating environment.
The United States is the primary contributor to regional growth, hosting many of the world's leading pharmaceutical and biotechnology companies, as well as a large concentration of CDMOs. Strict regulatory oversight by agencies such as the U.S. FDA drives continuous investment in advanced aseptic processing technologies.
North America also benefits from a mature research and development ecosystem and significant capital expenditure on facility upgrades and modernization. Manufacturers in the region prioritize automation, closed systems, and single-use technologies to maintain compliance and operational excellence.
While other regions-including Asia Pacific and Latin America-are expanding manufacturing capacity and regulatory oversight, North America is expected to remain the benchmark region that sets technological, regulatory, and operational standards for the global ATS market.
Competitive Landscape and Key Players
The aseptic transfer system market is characterized by the presence of specialized ATS manufacturers, cleanroom solution providers, and global pharmaceutical equipment suppliers. Key players operating in the market include:
ABC Transfer
Aseptic Group
Cape-Europe
Castus GmbH & Co. KG
Central Research Laboratories
JCE Biotechnology
Sartorius AG
STERIS plc
EMA Sinergie S.p.A.
Pharmalab India Private Limited
Inos
AST, LLC
Steriline S.r.l.
Ortner Reinraumtechnik
Other prominent players
Each company has been profiled in the market research report based on company overview, financial performance, business strategies, product portfolio, key business segments, and recent developments. Competitive differentiation is driven by technological innovation, system reliability, regulatory compliance capabilities, and integration with automated manufacturing environments.
Key Developments
February 2025: Getinge introduced its DPTE-FLEX, a manually operated, externally openable transfer port designed to enhance safety, efficiency, and compliance in pharmaceutical manufacturing. The solution enables secure, gloveless transfers and reduces contamination risks by minimizing human intervention. When combined with a sleeveless DPTE-BetaBag, the system eliminates the need for traditional glove ports.
January 2025: CPC (Colder Products Company), part of Dover, launched its MicroCNX Nano Series aseptic connectors, specifically designed to simplify closed sterile processing in cell and gene therapy applications. The new connectors address the growing demand for scalable, contamination-free transfer solutions in advanced therapy manufacturing.
Investment Landscape and Future Outlook
The aseptic transfer system market continues to attract strong investment interest due to its essential role in sterile manufacturing. As pharmaceutical pipelines increasingly focus on biologics, injectables, and advanced therapies, demand for reliable and compliant ATS solutions is expected to rise steadily.
Although upfront costs remain relatively high, long-term returns are driven by improved product quality, reduced contamination risk, fewer batch failures, and enhanced regulatory compliance. Manufacturers that invest early in advanced ATS technologies are likely to gain competitive advantages through operational efficiency and faster regulatory approvals.
The future of the ATS market will be shaped by continued innovation in automation, digital monitoring, single-use technologies, and sustainable system designs.
Explore our report to uncover in-depth insights -
https://www.transparencymarketresearch.com/aseptic-transfer-system-market.html
Market Segmentation Overview
By System Type
Liquid Transfer System
Solid Transfer System
By Usability
Single Use
Multiple Use
By Transfer Type
Port
Portbags
Others
By End-user
Pharmaceutical & Biotech Manufacturers
Contract Development & Manufacturing Organizations (CDMOs)
Others
By Region
North America
Europe
Asia Pacific
Latin America
Middle East & Africa
Why Buy This Report?
Understand current and future market size, trends, and growth opportunities
Gain insights into regulatory drivers and technological advancements
Analyze competitive strategies of leading ATS suppliers
Identify high-growth segments and regional investment hotspots
Support strategic planning with comprehensive, data-driven insights
Buy this Premium Research Report to explore detailed market trends -
https://www.transparencymarketresearch.com/checkout.php?rep_id=74065<ype=S
FAQs
1. What is the current size of the aseptic transfer system market?
The market was valued at US$ 1.5 billion in 2024.
2. What is the expected CAGR of the market?
The market is expected to grow at a CAGR of 6.7% from 2025 to 2035.
3. Which system type dominates the market?
Liquid transfer systems dominate the market, holding 61.4% share in 2024.
4. Which region leads the global market?
North America leads with a 35.8% revenue share.
5. What are the key growth drivers?
Rising production of sterile biologics, stringent regulatory requirements, and increasing adoption of single-use aseptic technologies.
Explore Latest Research Reports by Transparency Market Research:
Bioprocess Validation Market - https://www.transparencymarketresearch.com/bioprocess-validation-market.html
Thyroid Cancer Diagnostics Market - https://www.transparencymarketresearch.com/thyroid-cancer-diagnostics-market.html
Plasmid Purification Market - https://www.transparencymarketresearch.com/plasmid-purification-market.html
Ovarian Cancer Diagnostics Market - https://www.transparencymarketresearch.com/ovarian-cancer-diagnostics-market.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com
Follow Us: LinkedIn| Twitter| Blog | YouTube
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Aseptic Transfer System Market to Reach USD 3.0 Billion by 2035, Driven by Rising Sterile Biologics Production and Stringent Contamination-Control Regulations | TMR here
News-ID: 4317568 • Views: …
More Releases from Transparency Market Research
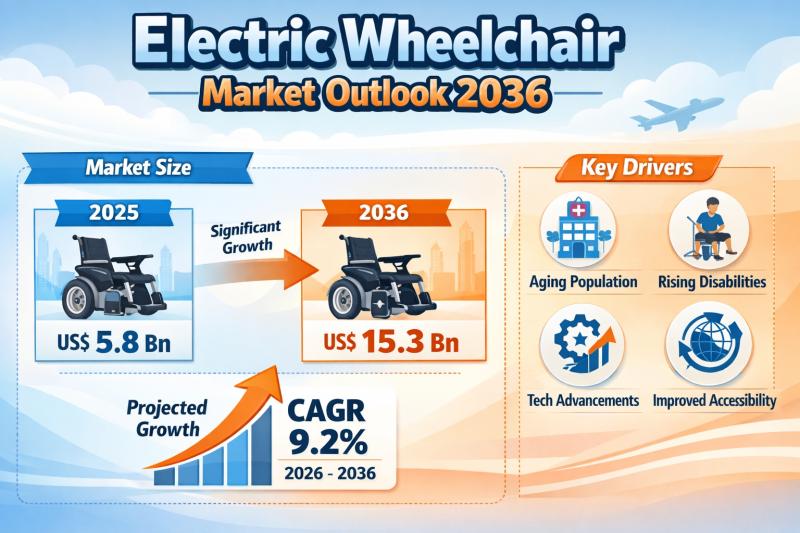
Electric Wheelchair Market Expanding at 9.2% CAGR Through 2036 - By Control Type …
The global electric wheelchair market continues to demonstrate strong and sustained growth, fueled by demographic transitions, technological innovation, and expanding healthcare access worldwide. Valued at US$ 5.8 billion in 2025, the market is projected to reach US$ 15.3 billion by 2036, expanding at a compound annual growth rate (CAGR) of 9.2% from 2026 to 2036.
Discover essential conclusions and data from our Report in this sample -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=4198
This robust trajectory reflects rising…
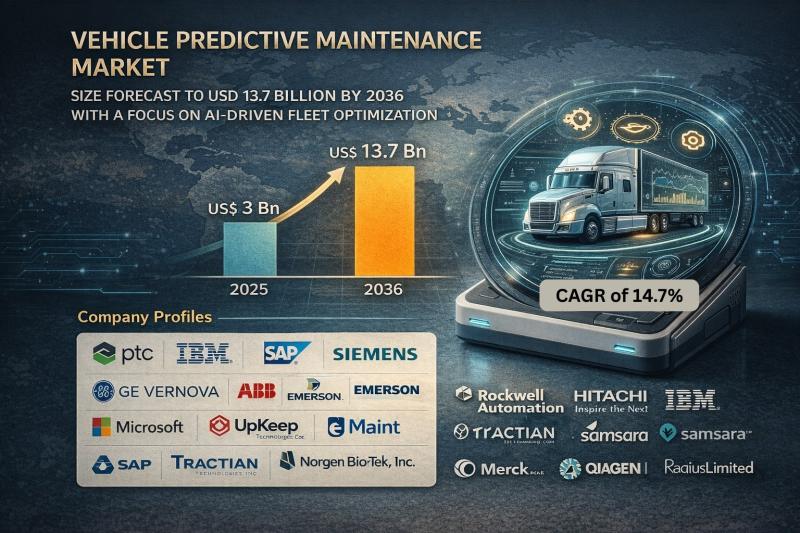
Vehicle Predictive Maintenance Market Size Forecast to USD 13.7 Billion by 2036 …
Vehicle Predictive Maintenance Market Outlook 2036
The global vehicle predictive maintenance market was valued at USD 3 Billion in 2025 and is projected to reach USD 13.7 Billion by 2036, expanding at a robust CAGR of 14.7% from 2026 to 2036. Market growth is driven by increasing adoption of connected vehicles, rising fleet digitalization, advancements in AI-driven analytics, and growing emphasis on minimizing vehicle downtime and maintenance costs.
👉 Get your sample…
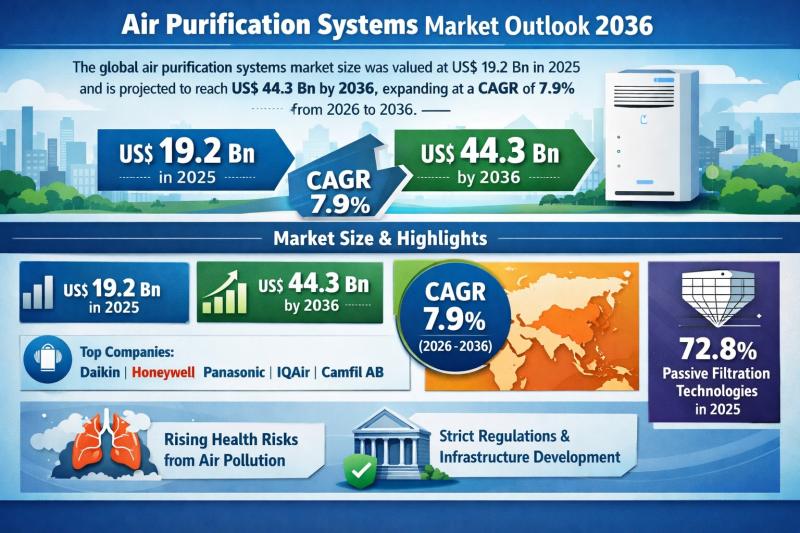
Global Air Purification Systems Market to Reach USD 44.3 Billion by 2036 at 7.9% …
The global Air Purification Systems Market was valued at US$ 19.2 Bn in 2025 and is projected to expand to US$ 44.3 Bn by 2036, registering a compound annual growth rate (CAGR) of 7.9% from 2026 to 2036. The market's upward trajectory reflects the structural shift in indoor air quality (IAQ) management, moving from discretionary consumer spending to mission-critical infrastructure investment.
With historical data available from 2021 to 2024, the industry…
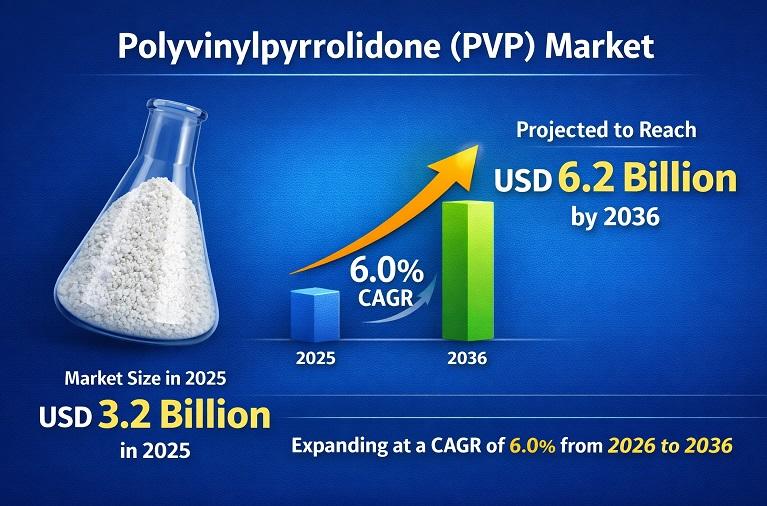
Polyvinylpyrrolidone (PVP) Market to Reach USD 6.2 Billion by 2036 Driven by Pha …
The Polyvinylpyrrolidone (PVP) Market was valued at around US$ 3.2 billion in 2025 and is projected to reach approximately US$ 6.2 billion by 2036, expanding at a steady CAGR of about 6.0% during the forecast period. This growth is primarily driven by rising demand from the pharmaceutical industry, where PVP is widely used as a tablet binder, solubilizer, and stabilizer, along with increasing consumption in cosmetics and personal care products…
More Releases for ATS
Geniee International Joins ATS Singapore 2025
Geniee International, a global leader in programmatic advertising and monetization technology, is proud to announce its participation as an official sponsor of ATS Singapore 2025, taking place on 2 July 2025 at Sands Expo & Convention Centre.
As part of its sponsorship, Geniee will be hosting a dedicated networking space at Booth No. 17, where attendees are invited to meet the team, exchange ideas, and explore potential collaborations. Visitors can expect…
Applicant Tracking System (ATS) Market To Witness Astonishing Growth
Coherent Market Insights recently conducted an industry analysis on the worldwide Applicant Tracking System (ATS) market, analyzing the market's future and current situation. The research also provides insights and updates on the relevant market sectors for the projected year of 2023-2030.
The key goals of this study were to assess the magnitude of a range of various categories and industries and to forecast which trends would gain traction over the next…
ATS Fertilizer Market to Witness Robust Expansion by 2025
LP INFORMATION recently released a research report on the ATS Fertilizer market analysis and elaborate the industry coverage, current market competitive status, and market outlook and forecast by 2025. Moreover, it categorizes the global ATS Fertilizermarket by key players, product type, applications and regions,etc.
The main objective of this market research is to help the readers understand the structure of ATS Fertilizermarket, market definition, overview, industry opportunities and trends, investment…
Benefits of ATS for an agency
Before getting into the benefits of ATS, let me tell you what an ATS is. In simple terms, an ATS (Applicant Tracking System) is a software program or tool that helps a recruiter through every step of the process of hiring candidates from job postings, job applications to scheduling interviews, sending automated emails to candidates all the way till choosing the right candidate for the job.
Basically, ATS is the technology…
Applicant Tracking System (ATS) Market To Witness Astonishing Growth
Applicant Tracking System (ATS) Market report plays very noteworthy role to achieve business growth and success in this competitive market place for industry. Best practice models and research methodologies are implemented in this report to give comprehensive market analysis with accurate market segmentation and insights. This market research report provides important and meaningful market insights for the business by taking into consideration various factors. It provides market data for several…
Applicant Tracking System (ATS) In Higher Education 2017-2021
Applicant Tracking System (ATS) - ATS are applications that manage recruitment process of an organization. They collect resumes in database and give recruiters an automated process of hiring from sourcing to hiring activities. They filter applications automatically based on given criteria such as keywords, skills, former employers, years of experience, and schools attended. This has caused many candidates to adapt resume optimization techniques, similar to those used in search engine…
