Press release
Omics-Based Clinical Trials Market Drivers Include Advanced Biomarker Discovery and Patient Stratification
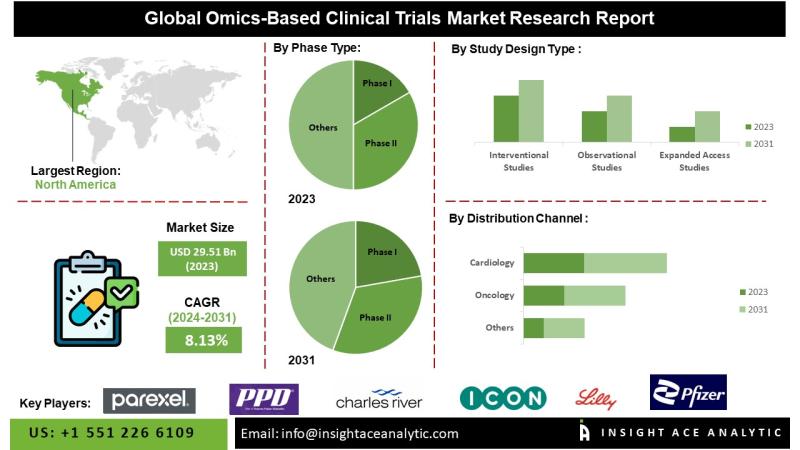
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Omics-Based Clinical Trials Market - (By Phase Type (Phase I, Phase II, Phase III, and Phase IV), By Study Design Type (Interventional Studies, Observational Studies, and Expanded Access Studies), By Indication Type (Oncology, Cardiology, Respiratory Diseases, Skin Diseases, CNS Diseases, Immunology, Genetic Diseases, and Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."According to the latest research by InsightAce Analytic, the Global Omics-Based Clinical Trials Market is valued at US$ 29.51 Bn in 2023, and it is expected to reach US$ 54.42 Bn by 2031, with a CAGR of 8.13% during the forecast period of 2024-2031.
Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/2355
The Omics-Based Clinical Trials market represents a specialized and rapidly evolving segment within the healthcare industry, emphasizing the utilization of advanced omics technologies-such as genomics, proteomics, metabolomics, and transcriptomics-in clinical research design and execution. These technologies enable comprehensive analysis of biological molecules and their interactions, providing enhanced insights into disease mechanisms, biomarker discovery, and personalized therapeutic strategies.
Incorporating omics data into clinical trial frameworks allows for more precise patient stratification, increased efficiency in drug development processes, and optimized trial outcomes. The market encompasses a wide array of stakeholders, including pharmaceutical and biotechnology companies, contract research organizations (CROs), academic research institutions, and technology providers, all collaborating to advance precision medicine and elevate patient care standards.
Key factors driving market growth include rising investments by leading pharmaceutical companies to enhance research productivity, growing demand for personalized medicine enabled by omics-based trials, and the increasing global prevalence of chronic diseases. The escalating incidence of cancer and other long-term conditions further underscores the need to identify novel biological targets that can serve as both clinical biomarkers and therapeutic intervention points.
List of Prominent Players in the Omics-Based Clinical Trials Market:
• Parexel International Corporation
• Pharmaceutical Product Development (PPD)
• Charles River Laboratory
• ICON plc
• SGS SA
• Eli Lilly and Company
• Pfizer Inc.
• Covance Inc.
• Novo Nordisk
• Rebus Bio,
• Other Prominent Players
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2025-04
Market Dynamics
Drivers:
The expansion of the omics-based clinical trials market is predominantly driven by ongoing advancements in omics technologies, particularly genomics and proteomics. These innovations have substantially enhanced the understanding of disease mechanisms at the molecular level, facilitating the identification of specific biomarkers and genetic variations that enable the development of personalized treatment regimens and optimized clinical trial designs.
The growing global emphasis on precision medicine further fuels market growth, as healthcare providers and patients increasingly demand therapies tailored to individual genetic profiles. By enabling comprehensive molecular profiling, omics-based approaches support more accurate patient stratification and the development of targeted therapeutic strategies.
Challenges:
Despite strong growth prospects, the market faces several challenges. The high costs associated with implementing omics technologies-including sophisticated instrumentation, specialized reagents, and skilled personnel-pose significant financial barriers, particularly for smaller organizations and academic institutions. Additionally, the large and complex datasets generated require advanced bioinformatics platforms and specialized analytical expertise, with insufficient computational resources or trained staff potentially delaying research progress. Regulatory and ethical considerations, including patient privacy, informed consent, and compliance with evolving regulatory frameworks, further complicate the execution of omics-driven clinical trials.
Regional Trends:
North America currently dominates the global omics-based clinical trials market, supported by substantial R&D investments, the presence of leading pharmaceutical and biotechnology companies, and a strong intellectual property environment. Concurrently, the Asia Pacific region is emerging as the fastest-growing market, driven by increased investment in omics research, cost-efficient trial execution, and access to a large and diverse patient population.
The region's expanding clinical research infrastructure, coupled with the growing presence of multinational pharmaceutical companies and contract research organizations (CROs), has accelerated market development. Numerous organizations are strategically expanding their operations in the region to leverage these favorable conditions and advance omics-based research initiatives.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customisation/2355
Recent Developments:
• In Jan 2023, Rznomics Inc. and Charles River Laboratories International, Inc. entered into a contract development and manufacturing organization (CDMO) partnership for viral vectors. By capitalizing on Charles River's expertise in viral vector CDMO, Rznomics intends to commence clinical development for liver cancer patients using its RNA-based anticancer gene therapy.
• In Jan 2022, Pfizer Inc. and BioNTech SE commenced a clinical trial to assess the safety, tolerance, and immune response of an Omicron-derived vaccination in healthy persons aged 18 to 55. The trial included three groups that investigated various dosages of the existing Pfizer-BioNTech COVID-19 vaccine or a vaccination based on the Omicron variant.
• In February 2021, Parexel and Neo Genomics developed a precision medicine strategic alliance with the goal of improving study designs and speeding up patient matching in cancer clinical trials.
Segmentation of Omics-Based Clinical Trials Market-
By Phase Type -
• Phase I
• Phase II
• Phase III
• Phase IV
By Study Design Type -
• Interventional Studies
• Observational Studies
• Expanded Access Studies
By Indication Type -
• Oncology
• Cardiology
• Respiratory Diseases
• Skin Diseases
• CNS Diseases
• Immunology
• Genetic Diseases
• Others
By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• Southeast Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
Read Overview Report- https://www.insightaceanalytic.com/report/omics-based-clinical-trials-market/2355
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: https://www.insightaceanalytic.com/
Tel : +1 607 400-7072
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Omics-Based Clinical Trials Market Drivers Include Advanced Biomarker Discovery and Patient Stratification here
News-ID: 4312817 • Views: …
More Releases from Insightace Analytic Pvt Ltd.
Automotive Lead Acid Battery Market Strategic Growth Drivers and Outlook 2026 to …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Lead Acid Battery Market Size, Share & Trends Analysis Report By Product (SLI and Micro-Hybrid Batteries), Type (Flooded, Enhanced Flooded, and VRLA), Customer Segment (OEM and Aftermarket), End User (Passenger Car, Light Commercial Vehicles, Heavy Commercial Vehicles, Two-Wheeler, and Three-Wheeler), and Application (Hybrid Vehicles, Electric Vehicles, Light Motor Vehicles, and Heavy Motor Vehicles)- Market…
Automotive Interior Market Investment Opportunities and Forecast 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Interior Market- (By Component Type (Center Stack, Head-up Display, Instrument Cluster, Rear Sear Entertainment, Dome Module, Headliner, Seat, Interior Lighting Door Panel, Center Console, Adhesives & Tapes, Upholstery, Others), By Material (Leather, Fabric, Vinyl, Wood, Glass Fiber Composite, Carbon Fiber Composite, Metal), By Level of Autonomy (Semi-Autonomous, Autonomous, Non-Autonomous),By Electric Vehicle (Battery Electric Vehicle…
Artificial General Intelligence Market Future Landscape and Industry Evolution 2 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Artificial General Intelligence (AGI) Market - (By Type of Offering (Hardware, Software and Service), Type of Technology (Machine Learning, Deep Learning, Natural Language Processing and Robotics), Mode of Deployment (Cloud-based, On Premise and Web-based), Type of AI (Weak AI, Strong AI and Superintelligence), Type of Processing (Image, Text and Voice Processing), Company Size (SMEs and…
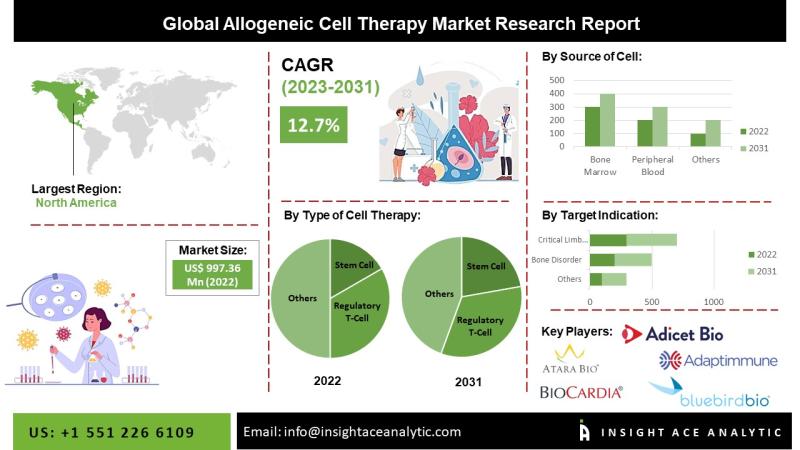
Allogenic Cell Therapies Market Revenue Trends and Growth Potential 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Allogenic Cell Therapies Market- by Cell Type(Cardiosphere-Derived Cells (CDCs), Fibroblasts, T-cells, Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs) and Others),Tissue Source(Skin, Blood, PBC, BM and Others), Indication (Acute graft-versus-host disease (GVHD), Chronic Ulcers and Diabetic Foot Ulcers, Osteoarthritis, Crohn's Disease, Cardiovascular Disease, Solid Tumors/Cancers and Others (Alzheimer's Disease, etc.)), Trends, Industry Competition Analysis, Revenue…
More Releases for Phase
Three-Phase Hybrid Inverter Market Efficient and Reliable Power Conversion Solut …
Global Three-Phase Hybrid Inverter Market Overview:
The Three-Phase Hybrid Inverter market is a broad category that includes a wide range of products and services related to various industries. This market comprises companies that operate in areas such as consumer goods, technology, healthcare, and finance, among others.
In recent years, the Three-Phase Hybrid Inverter market has experienced significant growth, driven by factors such as increasing consumer demand, technological advancements, and globalization. This growth…
Clinical Trials by Phase (Phase I, Phase II, Phase III, Phase IV) Market Forecas …
A clinical trial is a research study, where a group of people is given a test or treatment. Clinical trials study the safety and efficacy of tests and treatments. If the test or treatment is safe and meets regulatory requirements, then it is approved as a standard of care.
Download Sample Copy at https://www.theinsightpartners.com/sample/TIPRE00006203/?utm_source=OpenPR&utm_medium=10379
Key Players Analysis:
IQVIA
Parexel International Corporation
Charles River Laboratories
ICON plc
SGS SA
Chiltern International Ltd
Syneos Health
PRA Health Sciences
Wuxi AppTec Inc
Pharmaceutical Product Development,…
Digital Phase Shifters Market
Digital Phase Shifters Market A recently identified vacuum in the literature about the creation of digital phase shifters for modern communication systems is attempted to be filled in the book Design of Digital Phase Shifters for Multipurpose Communication Systems. By significantly reducing RF power consumption and improving noise immunity, directed beams enhance the development of new-generation mobile communication systems. In this regard, digital phase shifters in particular, which are part…
COVID-19 - Pipeline Analysis 2020 for Global Market | Emphasis on Products cover …
COVID-19 (also known as Anderson COVID-19)?is a viral disease caused by RNA virus, SARS-CoV-2 or commonly known as corona virus. These viruses can cause respiratory, enteric, hepatic, and neurologic diseases. At the end of 2019, a new coronavirus was identified as the cause of a cluster of pneumonia cases in Wuhan, China. It rapidly spread, resulting in an epidemic throughout China, followed by an increasing number of cases in other…
Power Metering Market Information by type (smart, digital, analog), by phase (si …
Power Metering Market Information by type (smart, digital, analog), by phase (single phase, three phase) by application (residential, commercial and industrial) and Region - Forecast to 2022
The report for Global Power Metering Market of Market Research Future comprises of extensive primary research along with the detailed analysis of qualitative as well as quantitative aspects by various industry experts, key opinion leaders to gain the deeper insight of the market and…
HIV Vaccines Market Perceive Aggrandized Growth at a CAGR of 11.48% Till 2023 | …
HIV Vaccines Market Report Added on MarketResearchFuture.com with Overall Analysis. Key developments and Strategies Cover in this Report. The Market for Expected to Grow Globally Over the CAGR of 5 % During the Period 2018 to 2027 from USD 2,702.3 Billion in 2027.
HIV Vaccines Market - Segmentation
The global HIV vaccines market has been segmented on the basis of basis of antibiotics, type, and lastly, region.
Antibiotics have been segmented into dicloxacillin,…