Press release
HIV Or HB Or HCV Test Kits Market to hit USD$ 8842.48 million by 2031: Infectious Disease Testing Globally | Biopanda Reagents Ltd; Nanjing Synthgene Medical Technology Co., Ltd.; AccuBioTech Co., Ltd.; BioMérieux SA; QIAGEN
HIV/HBV/HCV Test Kits Market size was valued at USD 5722.49 million in 2022, and it is projected to reach USD 8842.48 million by the end of 2031, expected to grow at a CAGR of 5.7% during the forecasting period (2025-2031).Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://datamintelligence.com/download-sample/hiv-hbv-hcv-test-kits-market?kb
United States: Recent Industry Developments
✅ November 2025: Abbott Laboratories launched a rapid multiplex test kit capable of simultaneously detecting HIV, HBV, and HCV with enhanced accuracy.
✅ October 2025: Quest Diagnostics expanded point-of-care testing services utilizing next-gen viral load test kits for early infection detection.
✅ September 2025: FDA approved new home-testing kits for HIV and Hepatitis B/C to improve accessibility and early diagnosis.
Japan: Recent Industry Developments
✅ November 2025: Fujirebio introduced advanced immunoassay kits with improved sensitivity for HBV and HCV detection in clinical settings.
✅ October 2025: Sysmex Corporation expanded its portfolio with integrated molecular diagnostic kits targeting co-infections of HIV and hepatitis viruses.
✅ September 2025: Japanese research institutions developed AI-assisted platforms to enhance interpretation accuracy of viral test results.
GCC: Recent Industry Developments
✅ November 2025: GCC health ministries launched regional screening programs using rapid HIV/HBV/HCV test kits to combat infectious diseases.
✅ October 2025: Local manufacturers partnered with global diagnostic firms to increase availability of affordable test kits across the Gulf.
✅ September 2025: UAE invested in healthcare infrastructure upgrades, integrating next-gen viral test technologies in public hospitals.
List of Top Key Player:
F. Hoffmann-La Roche Ltd; Bio-Rad Laboratories Inc.; Abbott Laboratories; Meridian Bioscience, Inc.; Biopanda Reagents Ltd; Nanjing Synthgene Medical Technology Co., Ltd.; AccuBioTech Co., Ltd.; BioMérieux SA; QIAGEN; Hologic Inc; and Creative Diagnostics.
Industry Developments:
1. Rapid diagnostic tests (RDTs) remain the largest segment due to ease of use and low cost, while molecular tests (PCR/NAAT) are gaining share because of higher sensitivity, especially for early infection and treatment monitoring.
Key technology and product developments
1. Multiplex and bundled panels: WHO prequalified the first bundled set of three rapid diagnostic tests able to simultaneously detect HIV, HBV and syphilis for antenatal care, marketed as the Determine Antenatal Care Panel, marking a milestone for integrated maternal screening and "triple elimination" efforts.
2. Point‐of‐care HCV RNA: The FDA granted marketing authorization to Cepheid's Xpert HCV test on the GeneXpert Xpress platform, the first point‐of‐care HCV RNA test usable in CLIA‐waived settings, enabling diagnosis and linkage to treatment in a single visit using fingerstick samples.
3. Molecular and viral‐load tests: New and next‐generation quantitative HCV viral‐load assays (e.g., from Roche) have been cleared/approved to support treatment response monitoring in chronic HCV.
WHO and guideline‐driven integration
1. WHO's Global HIV, Hepatitis and STIs Programme continues to emphasize integrated testing, with updated materials and a 2025 hepatitis programme overview linking HBV/HCV testing expansion to elimination goals.
2. A WHO self‐testing implementation toolkit published in early 2025 provides operational guidance for HIV and HCV (plus syphilis) self‐testing, supporting scale‐up of lay‐user kits and decentralized models.
Emerging themes: access, POC and procurement
1. Point‐of‐care and self‐testing: Market analyses highlight point‐of‐care RDTs and self‐tests as critical for reaching high‐risk and marginalized populations, cutting time to diagnosis and reducing reliance on central labs.
2. Public tenders and integrated panels: Government procurements, such as a 2025 state tender in India for rapid HIV/syphilis/HBV/HCV kits, illustrate how buyers are moving toward multi‐analyte panels to support broad screening in public health programmes
Forecast Projection:
The Global HIV/HBV/HCV Test Kits Market is poised for significant growth between 2025 and 2031. In 2024, the market maintained a steady upward trajectory, and with strategic initiatives by leading players accelerating adoption, the market is expected to soar throughout the forecast period. Companies leveraging these trends are well-positioned to capture emerging opportunities and maximize revenue potential.
Market Intelligence Research Process:
The HIV/HBV/HCV Test Kits Market research report by DataM Intelligence combines primary and secondary data to deliver deep, actionable insights. It examines the full spectrum of factors shaping the industry, from government regulations and market conditions to competitive dynamics, historical trends, technological breakthroughs, upcoming innovations, and potential challenges. This comprehensive analysis not only highlights growth prospects but also identifies barriers, equipping businesses to navigate market volatility and capitalize on emerging opportunities.
Buy Now & Get 30% OFF - Grab 50% OFF on 2+ reports: https://www.datamintelligence.com/buy-now-page?report=hiv/hbv/hcv-test-kits-market
Key Segmentation:
By Test Type: (Rapid Test Kits, Assay Based Test Kits)
By Sample Type: (Saliva, Blood, Urine)
By End-User: (Hospitals, Diagnostic Laboratories, Clinics, Government Organizations and NGO's, Others)
Growth Regional Analysis:
⇥ North America (U.S., Canada, Mexico)
⇥ Europe (U.K., Italy, Germany, Russia, France, Spain, The Netherlands and Rest of Europe)
⇥ Asia-Pacific (India, Japan, China, South Korea, Australia, Indonesia Rest of Asia Pacific)
⇥ South America (Colombia, Brazil, Argentina, Rest of South America)
⇥ Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of Middle East & Africa)
Key regulatory approvals (HIV / HBV / HCV diagnostics)
HIV diagnostics:
✅ The FDA approved the INSTI HIV Self Test via PMA in October 2025, authorizing a single‐use rapid self‐test for HIV‐1 antibodies that can be distributed for lay use in non‐laboratory settings.
✅ FDA's 2025 biological device approvals list also includes updated HIV combo assays (antigen/antibody and NAT) for diagnosis and viral‐load monitoring on automated analyzers, reinforcing lab‐based testing capacity.
HCV diagnostics:
✅ On 26 June 2024 (driving implementation through 2025), the FDA permitted marketing of Cepheid's Xpert HCV RNA test on the GeneXpert Xpress platform, the first HCV RNA assay authorized for use in CLIA‐waived point‐of‐care settings with fingerstick blood and ~1‐hour turnaround.
✅ National policy updates, such as Australia's 2025 National Hepatitis C Testing Policy, explicitly recognize and approve point‐of‐care HCV antibody and RNA tests under the TGA, encouraging wider decentralized use.
Integrated/other approvals:
✅ On 10 July 2025, WHO prequalified the first bundled set of three HIV/hepatitis B/syphilis rapid diagnostic tests for antenatal care (Determine Antenatal Care Panel), supporting integrated triple‐elimination strategies.
Get Customization in the report as per your requirements: https://datamintelligence.com/customize/hiv-hbv-hcv-test-kits-market
Point‐of‐care molecular HCV tests authorized/used in 2025
Cepheid Xpert HCV RNA (GeneXpert Xpress):
✅ FDA marketing authorization with CLIA waiver enables use in substance‐use clinics, correctional facilities, syringe‐service programs, EDs, urgent care, and doctor's offices, supporting "test‐and‐treat in one visit" models.
✅ The assay detects all major HCV genotypes from fingerstick blood in about an hour and is specifically positioned for active case finding at point of care.
Other POC molecular HCV use cases:
✅ National hepatitis testing policy in Australia notes that both POC antibody and RNA tests have TGA approval, and recommends their use to expand testing among people who decline traditional lab‐based testing
Request 2 Days Free Trials with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription?kb
Procurement, supply and value‐chain developments
WHO‐guided procurement:
✅ WHO outlines criteria for selecting/purchasing HIV, HBsAg and HCV in vitro diagnostics (sensitivity, specificity, invalid rates) and links eligibility for WHO and donor procurement to WHO prequalification status.
✅ A 2025 global reporting framework for HIV, viral hepatitis and STIs reinforces use of WHO‐prequalified test lists for national procurement planning.
Local production and supply‐chain resilience:
✅ WHO and the Global Fund highlight a shift toward local manufacturing in Africa: by mid‐2025, African‐produced HIV medicines and rapid tests are beginning to enter national programmes, with Codix Bio (Nigeria) sublicensed to manufacture HIV rapid diagnostic tests.
✅ WHO explicitly frames locally produced HIV RDTs as a way to increase affordability and reduce vulnerabilities and delays in diagnostic supply chains, urging advanced market commitments and fair procurement policies.
Programme‐level procurement trends:
1. Country tenders (for example, India's 2025 procurement of combined HIV/syphilis/HBV/HCV rapid tests) indicate a move toward multi‐analyte panels, which simplify logistics and improve coverage in public screening campaigns.
2. WHO also encourages adoption of HIV self‐tests to mitigate human‐resource gaps and buffer stock‐out risks for first‐line RDTs in national algorithms, integrating self‐testing into procurement and distribution plans.
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release HIV Or HB Or HCV Test Kits Market to hit USD$ 8842.48 million by 2031: Infectious Disease Testing Globally | Biopanda Reagents Ltd; Nanjing Synthgene Medical Technology Co., Ltd.; AccuBioTech Co., Ltd.; BioMérieux SA; QIAGEN here
News-ID: 4310956 • Views: …
More Releases from DataM Intelligence 4 Market Research LLP
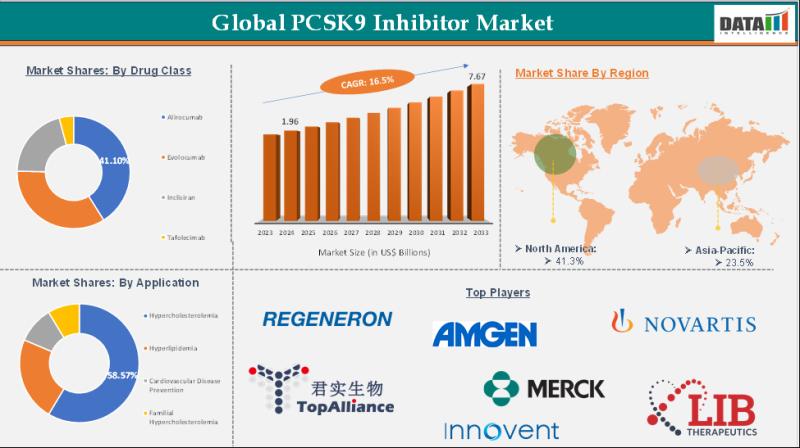
PCSK9 Inhibitor Market Set for Explosive Growth to USD 7.67 Billion by 2033, Led …
The PCSK9 Inhibitor Market reached USD 1.96 billion in 2024 and is expected to reach USD 7.67 billion by 2033, growing at a robust CAGR of 16.5% during the forecast period 2025-2033.
Market growth is driven by the rising prevalence of cardiovascular diseases, increasing demand for effective cholesterol-lowering therapies, and strong clinical outcomes from PCSK9 inhibitors like evolocumab and alirocumab. Advancements in monoclonal antibody technologies, expanding indications for hypercholesterolemia treatment, growing…

United States Data-centric Security Market Drivers: The Future of Privacy, Compl …
DataM Intelligence has published a new research report on "Data-centric Security Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Get a Sample PDF Of This Report (Get…
Automotive Semiconductor Market Set for Robust Growth to USD 86.73 Billion by 20 …
The Automotive Semiconductor Market reached USD 50.38 billion in 2024 and is expected to reach USD 86.73 billion by 2033, growing at a robust CAGR of 6.20% during the forecast period 2025-2033.
Market growth is driven by the rising adoption of electric vehicles, advanced driver-assistance systems (ADAS), and vehicle electrification technologies. Continuous innovation in power electronics, sensors, microcontrollers, and increasing integration of connectivity and automation features are further accelerating market expansion.
Get…

Future of Fiberglass Roving Market: Forecast, Challenges & Investment Opportunit …
DataM Intelligence has published a new research report on "Fiberglass Roving Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Get a Sample PDF Of This Report (Get…
More Releases for HCV
HIV/HBV/HCV Test Kits Market - Break the chain: Detect and prevent HIV, HBV, and …
Newark, New Castle, USA: The "HIV/HBV/HCV Test Kits Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
HIV/HBV/HCV Test Kits Market: https://www.growthplusreports.com/report/hivhbvhcv-test-kits-market/8774
This latest report researches the industry structure,…
Segmentation of Hepatitis C Virus (HCV) Antiviral Market: Trends, Opportunities, …
Hepatitis C Virus (HCV) Antiviral Market Overview:
Hepatitis C is a contagious liver disease caused due to hepatitis C virus. Hepatitis C damages and infects the liver. Hepatitis C is spread as the infected blood comes in contact with non-infected blood. Ranging in severity hepatitis C can cause acute and chronic hepatitis infection. Chronic hepatitis C is diagnosed by liver biopsy and blood tests. According to World Health Organization (WHO), globally…
Global Hepatitis C Virus (HCV) Market - Opportunities & Forecasts, 2020-2029
Dhirtek Business Research and Consulting recently released its most comprehensive research report to date on the global hepatitis c virus (hcv) market. Through extensive research, analysts have provided an extensive look into the market's drivers and restraints, and identified the key milestones and trends that will shape its future.
Primary and secondary research methods were used to create this in-depth report. Through the analysis of the research, Dhirtek was able to…
HCV Vehicles To Drive Automotive Turbocharger Hose Market Forecast Worldwide
An increase in manufacturing and sales of commercial vehicles will significantly influence the automotive turbocharger hose industry trends through the next few years. There is a strong demand for fuel-efficient passenger vehicles in countries like U.S., China, Germany, the UK, and France.
Various OEM participants are consistently working towards incorporating fuel-efficient engines to enhance the performance of the vehicles. Besides, there is an ease of access and abundant availability of turbocharger…
Hepatitis C Virus (HCV) Antiviral Market to Witness Comprehensive Growth by 2020
Inflammation of liver causes hepatitis. Hepatitis C is a contagious liver disease caused due to hepatitis C virus. Hepatitis C damages and infects the liver. Hepatitis C is spread as the infected blood comes in contact with non-infected blood. Ranging in severity hepatitis C can cause acute and chronic hepatitis infection. Chronic hepatitis C is diagnosed by liver biopsy and blood tests.
Get access to full summary @: http://www.persistencemarketresearch.com/market-research/hepatitis-c-virus-antiviral-market.asp
According to…
Global Hepatitis C Virus (HCV) Market Report: 2016 Edition
Hepatitis C Virus (HCV) is an infection which affects the liver which may lead to serious consequences if not taken care of. It spreads through infected blood transfusions and poorly sterilized medical equipments. The number of patients has increased significantly because its symptoms take time to be recognized before the patient progresses towards a clinically visible liver damage which worsens the situation since it may lead to liver damage or…