Press release
How to Build an Enoxaparin Plant: Investment, Approvals, & Cost Breakdown
Introduction - Overview of Enoxaparin Manufacturing Plant Setup:Establishing an enoxaparin manufacturing plant setup is a highly specialized and profitable opportunity within the pharmaceutical sector. Enoxaparin is a low molecular weight heparin (LMWH) widely used as an anticoagulant for preventing and treating blood clots. It requires advanced biochemical processing, strict sterile conditions, and compliance with global regulatory standards.
With increasing demand for anticoagulant therapies worldwide, this project-report-style guide explains the production process, plant setup needs, financial considerations, and operational requirements for launching an enoxaparin manufacturing facility.
Market Overview & Trends - Rising Demand for Anticoagulant Therapies:
The enoxaparin market is expanding due to growth in cardiovascular diseases, surgical procedures, trauma care, and deep vein thrombosis (DVT) prevention. Hospitals, clinics, and homecare settings are increasingly adopting LMWHs due to their efficacy and predictable pharmacokinetics. Market trends include the rise of biosimilar enoxaparin, improved purification technologies, sterile injectable formats, and demand from developing healthcare markets.
Challenges include stringent regulations, high production costs, and requirement for GMP-certified facilities. However, opportunities remain strong due to global healthcare expansion and rising incidence of clot-related disorders.
Get Your Sample Report Now: https://www.imarcgroup.com/enoxaparin-manufacturing-plant-project-report/requestsample
Report Coverage Highlights:
• Process Flow
• Land & Location
• Plant Layout
• Machinery
• Raw Materials
• Packaging
• Additional Needs
• Project Economics
• Financial Analysis
• Market Insights
Technical Aspects / Manufacturing Process - How Enoxaparin Is Produced:
The production of enoxaparin is a complex biochemical process derived from heparin extracted from porcine intestinal mucosa. The process involves:
• Heparin Sourcing & Preparation: Raw heparin undergoes extraction and purification.
• Depolymerization Process: Controlled chemical or enzymatic cleavage reduces molecular weight to produce enoxaparin.
• Purification: High-performance techniques such as chromatography remove impurities and achieve required molecular weight distribution.
• Formulation: The purified API is formulated into injectable solutions.
• Sterile Filtration & Filling: Solutions are processed in aseptic environments and filled into syringes, vials, or ampoules.
• Lyophilization (if applicable): Some formats may require freeze-drying for stability.
Quality control includes molecular weight profiling, anti-factor Xa potency tests, sterility tests, pyrogen checks, impurity analysis, and GMP documentation compliance.
Plant Setup Requirements - Machinery, Cleanrooms & Infrastructure:
A enoxaparin manufacturing plant setup requires advanced pharmaceutical infrastructure ensuring sterile and controlled environmental conditions.
Essential sections include:
• API processing area
• Purification zone (chromatography)
• Aseptic filling rooms
• Cleanrooms (ISO 5-8)
• HVAC systems
• Quality control and microbiology laboratories
• Packaging & labeling units
• Cold storage rooms
Key machinery includes reactors, filtration units, chromatography systems, lyophilizers, sterilizers, automated filling machines, air handling units (AHUs), vial/syringe filling lines, and QC testing equipment.
Safety and regulatory infrastructure must comply with GMP, WHO, FDA, EMA, and pharmacopoeia standards.
Project Economics - Capital Cost, Operating Cost & Financial Feasibility:
The enoxaparin manufacturing plant setup cost is high due to specialized sterile infrastructure, advanced purification systems, and compliance requirements. Capital costs include land, cleanroom construction, reactors, filtration systems, lyophilization units, packaging equipment, utilities, and working capital.
Operating expenses include raw heparin procurement, chemicals, skilled labor, sterile facility maintenance, QC testing, consumables, and regulatory compliance documentation.
Revenue opportunities include supplying hospitals, distributors, pharmaceutical partners, and global export markets. Profitability depends on stringent quality standards, certifications, contract manufacturing opportunities, and biosimilar market demand.
Risk Factors & Success Strategies - Quality Assurance & Regulatory Compliance:
Key risks include regulatory challenges, contamination risks, variability in raw heparin supply, and high infrastructure costs. Manufacturing enoxaparin also requires rigorous sterility assurance and pharmacovigilance.
Success strategies include adopting automated purification systems, maintaining strict GMP compliance, establishing long-term supply agreements for raw heparin, investing in advanced QC labs, and building strong regulatory documentation. Continuous staff training and consistent process validation are crucial for maintaining product safety and efficacy.
Request a Customized Project Report for Your Capacity: https://www.imarcgroup.com/request?type=report&id=9047&flag=C
How IMARC Group Supports Plant Setup:
IMARC Group helps investors and entrepreneurs establish a manufacturing plant by providing detailed market research, technical guidance, and financial feasibility analysis. Their reports outline process flow, machinery requirements, raw materials, project cost, profitability, and regulatory needs, offering a complete roadmap for setup. With expert consulting and customized solutions, IMARC ensures smoother planning, reduced risks, and faster project execution.
Conclusion - Feasibility and Long-Term Potential of Enoxaparin Manufacturing:
A enoxaparin manufacturing plant setup is a highly feasible and profitable venture for pharmaceutical investors with access to advanced technology and regulatory expertise. With rising global demand for anticoagulants and increasing healthcare spending, a well-designed enoxaparin production facility can offer strong long-term returns and global market reach.
FAQs - Enoxaparin Manufacturing Plant Setup:
1. What raw materials are needed for enoxaparin production?
A. Porcine intestinal mucosa (for heparin), depolymerization reagents, solvents, filtration consumables, and packaging materials.
2. What machinery is required for enoxaparin manufacturing?
A. Reactors, filtration units, chromatography systems, lyophilizers, sterile filling machines, AHUs, and QC laboratory equipment.
3. What factors influence the production cost of enoxaparin?
A. Raw heparin quality, purification efficiency, sterile infrastructure, regulatory compliance, and labor expertise.
4. How can manufacturers ensure product safety and regulatory compliance?
A. By implementing GMP-certified processes, ensuring aseptic conditions, conducting extensive QC tests, and adhering to FDA/EMA pharmacopoeial standards.
About Us:
IMARC Group is a leading global market research and management consulting firm. We specialize in helping organizations identify opportunities, mitigate risks, and create impactful business strategies.
Contact Us:
IMARC Group
134 N 4th St., Brooklyn, NY 11249, USA
Email: sales[@]imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: (+1-201971-6302)
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release How to Build an Enoxaparin Plant: Investment, Approvals, & Cost Breakdown here
News-ID: 4306597 • Views: …
More Releases from IMARC Group

Indonesia Battery Market to Surge to USD 4.4 Billion by 2034 at 11.72% CAGR - Re …
A Comprehensive Introduction to the Indonesia Battery Market Report
According to IMARC Group's report titled "Indonesia Battery Market Size, Share, Trends and Forecast by Technology, Application, and Region, 2026-2034" the report offers a comprehensive analysis of the industry, including market share, growth, trends, and regional insights.
Get Instant Access to the Free Sample (Corporate Email Required): https://www.imarcgroup.com/indonesia-battery-market/requestsample
Indonesia Battery Market Overview
The Indonesia battery market size was valued at USD 1.6 Billion in 2025.…

Thailand Cheese Market to Reach USD 31.0 Billion by 2033 | 11.80% CAGR | Get Fre …
Thailand Cheese Market Report Introduction
According to IMARC Group's report titled "Thailand Cheese Market Size, Share, Trends and Forecast by Source, Type, Product, Format, Distribution Channel, and Region, 2025-2033" the report offers a comprehensive analysis of the industry, including market share, growth, trends, and regional insights.
Free Sample Download PDF (Exclusive Offer on Corporate Email): https://www.imarcgroup.com/thailand-cheese-market/requestsample (Note: We are currently updating our reports to the 2026-2034 period. Click the link above to…

India Two-Wheeler Loan Market to Reach USD 14.55 Billion by 2033 | 6.43% CAGR | …
India Two-wheeler Loan Market Report Introduction
According to IMARC Group's report titled "India Two-Wheeler Loan Market Size, Share, Trends and Forecast by Type, Provider Type, Percentage Amount Sanctioned, Tenure, and Region, 2025-2033" the report offers a comprehensive analysis of the industry, including market share, growth, trends, and regional insights.
Free Sample Download PDF (Exclusive Offer on Corporate Email): https://www.imarcgroup.com/india-two-wheeler-loan-market/requestsample
Note : We are in the process of updating our reports to cover…
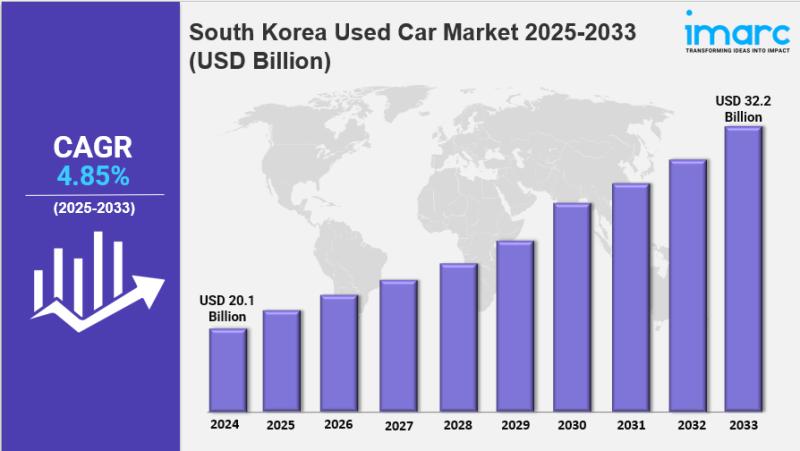
South Korea Used Car Market Size, Share, Industry Overview, Trends and Forecast …
IMARC Group has recently released a new research study titled "South Korea Used Car Market Report by Vehicle Type (Hatchback, Sedan, Sports Utility Vehicle, and Others), Vendor Type (Organized, Unorganized), Fuel Type (Gasoline, Diesel, and Others), Sales Channel (Online, Offline), and Region 2025-2033", offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios.
South Korea Used Car Market…
More Releases for Enoxaparin
Enoxaparin API Market Demand Driven by Healthcare Needs | Persistence Market Res …
✅ Market Overview: Trends, Statistics, and Growth Drivers
The Enoxaparin API market has gained substantial momentum in recent years, largely fueled by a surge in global demand for anticoagulant therapies. Enoxaparin, a low molecular weight heparin (LMWH), is widely used in the treatment of thrombotic conditions such as deep vein thrombosis (DVT), pulmonary embolism, and acute coronary syndromes. Its inclusion in the World Health Organization's list of essential medicines underlines…
Comprehensive Report on an Enoxaparin Manufacturing Plant Project: Cost and Reve …
Setting up an enoxaparin manufacturing facility necessitates a detailed market analysis alongside granular insights into various operational aspects, including unit processes, raw material procurement, utility provisions, infrastructure setup, machinery and technology specifications, workforce planning, logistics, and financial considerations.
IMARC Group's report titled "Enoxaparin Manufacturing Plant Project Report 2025: Industry Trends, Plant Setup, Machinery, Raw Materials, Investment Opportunities, Cost and Revenue" offers a comprehensive guide for establishing an enoxaparin manufacturing plant, covering…
Global Enoxaparin API Market Report: Innovations and Regional Analysis for 2024- …
Enoxaparin API Market Size
The global market for Enoxaparin API was estimated to be worth US$ 670 million in 2023 and is forecast to a readjusted size of US$ 1001.5 million by 2030 with a CAGR of 5.9% during the forecast period 2024-2030.
North American market for Enoxaparin API was valued at $ million in 2023 and will reach $ million by 2030, at a CAGR of % during the forecast period…
Enoxaparin Sodium Market : Size, Share, Growth, Analysis, Key Players, Revenue, …
Enoxaparin Sodium Market Size
The global Enoxaparin Sodium market was valued at US$ 3315 million in 2023 and is anticipated to reach US$ 5415 million by 2030, witnessing a CAGR of 7.1% during the forecast period 2024-2030.
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-6L9458/Global_Enoxaparin_Sodium_Market_Insights_and_Forecast_to_2028
Enoxaparin Sodium Market
Enoxaparin sodium is an anticoagulant medication (blood thinner). It is used to treat and prevent deep vein thrombosis (DVT) and pulmonary embolism (PE) including during pregnancy and following certain types of…
Enoxaparin Sodium Market Revenue, Insights, Overview, Outlook, Analysis | Valuat …
Enoxaparin Sodium Market
The global Enoxaparin Sodium market was valued at US$ 3044.5 million in 2022 and is anticipated to reach US$ 5354.7 million by 2029, witnessing a CAGR of 8.3% during the forecast period 2023-2029.
Get Free Research Report: https://reports.valuates.com/request/sample/QYRE-Auto-6L9458/Global_Enoxaparin_Sodium_Market_Insights_and_Forecast_to_2028
Enoxaparin Sodium Market Share
Europe is the largest market of Enoxaparin Sodium, with about 60% market share, followed by North America with about 17% market share. Currently, there are many players in…
Advancements in Enoxaparin: An Overview of Latest Developments and Trends
The global 𝗘𝗻𝗼𝘅𝗮𝗽𝗮𝗿𝗶𝗻 market is estimated to attain a valuation of by the end of 2031, states a study by Transparency Market Research (TMR).
The key objective of the TMR report is to offer a complete assessment of the global market including major leading stakeholders of the Enoxaparin industry. The current and historical status of the market together with forecasted market size and trends are demonstrated in the assessment in…
