Press release
Biological Safety Testing Market to Reach US$ 9.81 Billion by 2031 at 12.4% CAGR; North America Leads with 41% Share - Key Players: Thermo Fisher, Lonza, Eurofins, Charles River
The Global Biological Safety Testing Market was valued at US$ 4.32 billion in 2024 and is expected to reach US$ 9.81 billion by 2031, growing at a CAGR of 12.4% during the forecast period 2025-2031. Growth is driven by the rising production of biologics, vaccines, cell and gene therapies, and biosimilars, all of which require stringent safety evaluations to meet global regulatory standards. Increasing investments in biopharmaceutical R&D and the expansion of manufacturing facilities further support the adoption of advanced safety testing solutions.Biological safety testing encompasses sterility testing, bioburden analysis, viral safety assessments, endotoxin testing, residual host cell protein (HCP) analysis, and other essential quality checks that ensure the purity, safety, and efficacy of biological products. Continuous advancements in analytical methods, automation, and high-throughput technologies are enhancing accuracy and reducing turnaround times. As regulatory expectations strengthen and biologics pipelines expand worldwide, the demand for reliable biological safety testing is expected to accelerate across major healthcare and biopharma markets.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/biological-safety-testing-market?sai-v
The Biological Safety Testing Market refers to the industry that provides assays and services to ensure the safety, purity, and quality of biopharmaceutical products by detecting contaminants such as viruses, bacteria, and endotoxins.
Key Developments
✅ October 2025: Leading biopharmaceutical companies expanded end-to-end biological safety testing programs to support cell and gene therapy pipelines, increasing demand for mycoplasma, sterility, and viral safety assays.
✅ September 2025: U.S. contract research organizations (CROs) implemented high-throughput automated biosafety testing platforms to accelerate biologics release timelines and reduce human error.
✅ August 2025: European regulatory authorities issued updated safety guidelines for adventitious agent testing in ATMPs, prompting wider adoption of rapid PCR-based and next-gen sequencing (NGS) biosafety workflows.
✅ July 2025: Asia-Pacific biologics manufacturers invested in integrated biosafety labs with AI-driven contamination detection systems to enhance quality control for vaccines and recombinant therapeutics.
✅ May 2025: Global testing service providers launched next-generation viral vector safety testing solutions optimized for AAV, lentivirus, and mRNA-based therapeutics.
✅ March 2025: International biotech startups introduced rapid mycoplasma and endotoxin detection kits enabling real-time in-process monitoring in biologics manufacturing.
Mergers & Acquisitions
✅ November 2025: A major global biosafety testing company acquired a U.S.-based viral safety analytics firm to expand its capabilities in gene therapy quality assurance.
✅ August 2025: A European life sciences tools provider partnered with an Asian diagnostics company to co-develop high-speed biosafety testing systems for emerging biologics platforms.
✅ June 2025: A leading North American CRO acquired a microbial detection technology startup to integrate advanced rapid testing solutions into its biologics safety portfolio.
Key Players
Avance Biosciences | Cytovance Biologics | Eurofins Scientific | Lonza | Merck KGaA | Promega Corporation | Thermo Fisher Scientific | Toxikon | WuXi AppTec | Charles River Laboratories
Key Highlights
Avance Biosciences - Holds a 6.2% share, driven by its specialized genomic, molecular biology, and cell-based assay services supporting biopharmaceutical research and regulatory submissions.
Cytovance Biologics - Holds a 5.4% share, supported by its strong expertise in microbial and mammalian biologics manufacturing, process development, and CDMO capabilities.
Eurofins Scientific - Holds a 12.7% share, fueled by its broad portfolio of analytical testing, bioanalysis, and regulatory-compliant laboratory solutions for global biotech companies.
Lonza - Holds a 14.3% share, driven by its leadership in biologics manufacturing, advanced therapy production, and end-to-end drug development services.
Merck KGaA - Holds an 11.9% share, supported by its high-quality reagents, assay technologies, and comprehensive solutions for bioprocessing and biosafety testing.
Promega Corporation - Holds a 7.1% share, driven by its industry-leading molecular biology tools, assay technologies, and innovations in drug discovery support.
Thermo Fisher Scientific - Holds a 15.8% share, supported by its extensive instrumentation portfolio, analytical solutions, and growing dominance in biopharmaceutical testing and manufacturing services.
Toxikon - Holds a 4.6% share, recognized for its preclinical safety testing, toxicology services, and strong regulatory expertise.
WuXi AppTec - Holds a 10.5% share, driven by its full-service CRO/CMO platform, integrated drug discovery solutions, and global expansion in biologics and cell/gene therapy support.
Charles River Laboratories - Holds an 11.5% share, supported by its strong preclinical services, advanced safety assessment capabilities, and extensive global research infrastructure.
Purchase this report before year-end and unlock an exclusive 30% discount: https://www.datamintelligence.com/buy-now-page?report=biological-safety-testing-market?sai-v
(Purchase 2 or more Reports and get 50% Discount)
Market Drivers
- Increasing demand for biologics, vaccines, and cell & gene therapies requiring stringent safety and quality testing.
- Rising prevalence of chronic diseases driving the development of advanced biopharmaceuticals.
- Growing regulatory requirements from FDA, EMA, and WHO for contamination control and product safety.
- Rapid expansion of biopharmaceutical manufacturing and outsourcing to CROs and CDMOs.
- Advancements in analytical technologies, including high-sensitivity assays and rapid microbial detection systems.
- Increasing use of biological materials in drug development, tissue engineering, and regenerative medicine.
- Rising focus on ensuring product purity, sterility, and absence of mycoplasma, viruses, and endotoxins in biologics.
Industry Developments
- Launch of rapid, automated testing platforms for sterility, mycoplasma, and adventitious agent detection.
- Strategic partnerships between biotech firms and testing service providers to enhance biosafety testing capacity.
- Development of next-generation assay technologies including PCR-based and high-throughput screening methods.
- Expansion of global biological safety testing facilities to support growing biologics production.
- Increasing investment in digital quality systems and AI-enabled data analysis for biosafety workflows.
- Rising adoption of in vitro alternative testing models to meet ethical and regulatory requirements.
Regional Insights
North America - 41% share: "Driven by strong biopharmaceutical R&D, large-scale biologics production, and stringent regulatory frameworks."
Europe - 30% share: "Supported by advanced biotechnology ecosystems, strict biosafety regulations, and growing vaccine manufacturing."
Asia Pacific - 22% share: "Fueled by expanding biopharma manufacturing, government support for biotech innovation, and rising outsourcing activities."
Latin America - 4% share: "Boosted by growing pharmaceutical production and increasing adoption of standardized biosafety practices."
Middle East & Africa - 3% share: "Driven by expanding healthcare infrastructure and rising investment in biologics and vaccine development."
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/biological-safety-testing-market?sai-v
Key Segments
➥ By Product
Reagents and Kits: Essential consumables used for microbial detection, contamination analysis, and quality assurance across biopharma manufacturing and research environments.
Instruments: Includes automated analyzers, microbial detection systems, PCR instruments, and other advanced platforms supporting high-precision contamination testing.
Other Products: Encompasses culture media, lab accessories, software tools, and supporting materials used for routine and advanced microbial quality control workflows.
➥ By Application
Vaccines and Therapeutics: Used to ensure sterility, purity, and safety in the development and production of vaccines, biologics, and therapeutic formulations.
Cellular and Gene Therapy: Critical for contamination testing, aseptic validation, and quality monitoring in cell-based therapies, viral vectors, and gene modification processes.
Blood and Blood-Based Therapy: Ensures microbial safety in blood products, plasma-derived therapies, and transfusion-related processes.
Other Applications: Covers contamination testing in biosimilars, monoclonal antibodies, tissue engineering products, and broader biopharmaceutical manufacturing.
➥ By Test
Bioburden Tests: Evaluate the total number of viable microorganisms present in raw materials, intermediates, and final products.
Sterility Tests: Confirm the absence of viable microorganisms in sterile pharmaceutical products, ensuring compliance with regulatory standards.
Endotoxin Tests: Detect pyrogenic contaminants using LAL assays, recombinant factor C technology, or alternative endotoxin detection methods.
Other Tests: Includes mycoplasma testing, environmental monitoring assays, microbial limit tests, and rapid microbiological methods (RMM).
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biological Safety Testing Market to Reach US$ 9.81 Billion by 2031 at 12.4% CAGR; North America Leads with 41% Share - Key Players: Thermo Fisher, Lonza, Eurofins, Charles River here
News-ID: 4304853 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
Electrophysiology Market Growth Value US$22.63 Billion by 2033, Future of Rhythm …
Electrophysiology Market reached US$9.06 Billion in 2024 and is expected to reach US$22.63 Billion by 2033, growing at a CAGR of 10.7% during the forecast period 2025-2033
Get a Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://datamintelligence.com/download-sample/electrophysiology-market?kb
Latest Mergers and Acquisitions
• Ametek completed acquisition of LKC Technologies, a visual electrophysiology diagnostics firm, in early Feb 2026.
• Boston Scientific agreed to acquire SoniVie Ltd., a developer of ultrasound/EP…
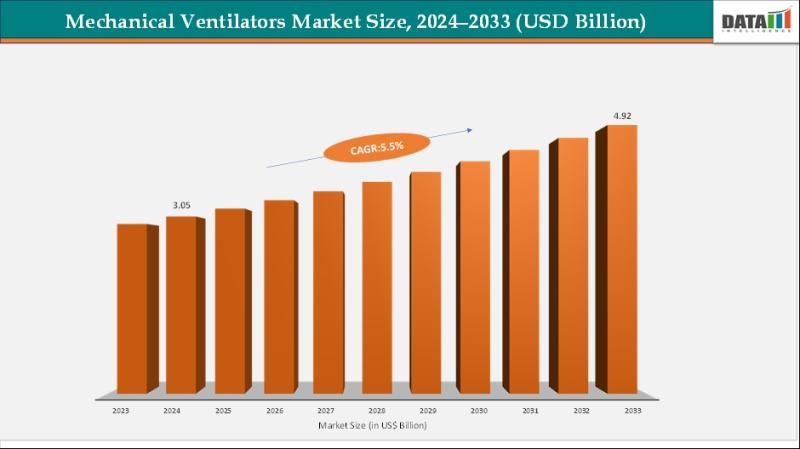
Mechanical Ventilators Market to Reach US$ 4.92 Billion by 2033 at 5.5% CAGR | N …
Mechanical Ventilators Market is projected to grow from US$ 3.05 billion in 2024 to US$ 4.92 billion by 2033, registering a CAGR of 5.5% during the forecast period of 2025 to 2033. The market is expanding steadily as the rising prevalence of chronic respiratory diseases such as chronic obstructive pulmonary disease and asthma increases the need for advanced respiratory support and critical care infrastructure across hospitals and home care environments.…

Organic Baby Food Market size is expected to reach USD 21.59 billion with a CAGR …
The global Organic Baby Food Market size was USD 8.7 billion in 2025 and is projected to grow from USD 9.56 billion in 2026 to USD 21.59 billion by 2034 at a CAGR of 10.72% during the 2026-2034 period. North America dominated the organic baby food market with a market share of 34.01% in 2025. Moreover, the organic baby food market size in the U.S. is projected to grow significantly,…

Sugar Free Beverages Market Size is Expected to reach USD 10.9 billion by 2031, …
The Global Sugar Free Beverages Market size reached USD 7.9 billion in 2022 and is expected to reach USD 10.9 billion by 2031 and is expected to grow with a CAGR of 4.2% during the forecast period 2024-2031. The beverage industry is experiencing a significant shift in consumer preferences towards healthier options, leading to the rise of sugar-free and low-sugar drinks.
Get a Free Sample PDF Of This Report (Get Higher…
More Releases for Test
Key Differences Between Megger Test, Tan Delta Test, and Hi-Pot Test for Electri …
Electrical insulation plays a critical role in ensuring the safety and efficiency of electrical systems. To assess the condition of insulation and identify potential issues, three common tests are used: the Megger test, Tan Delta test, and Hi-Pot test. Each test serves a unique purpose and provides valuable insights into the state of electrical insulation. Here's a closer look at the differences between these three essential tests.
Megger Test: Insulation Resistance…
Vitamin Test Market: Global Vitamin Test Analysis and Forecast (2023-2029)Vitami …
12.04.2024: Vitamin Test Market Overview
The development of companion diagnostic tools and advances in personalised treatment are driving considerable growth and revolution in the oncology Vitamin Test market. In the era of precision medicine, where healthcare is increasingly customised for individual individuals based on their own genetic and molecular profiles, this market segment is essential. Ongoing innovation and development define the oncology Vitamin Test market. To find particular biomarkers, genetic mutations,…
CAGR 8.1% Homecare Pregnancy Test Kits Market By Type of Test (Urine Test For H …
The Homecare Pregnancy Test Kits market report by Reports and Data provides an extensive overview of the vital elements of the Homecare Pregnancy Test Kits market and factors such as the drivers, restraints, latest trends, supervisory scenario, competitive landscape, technological advancements, and others. Further, it mentions the market shares associated with the market in terms of both value and volume along with the segmentation. Space-age industrial and digitalization tools are…
Home Safety Test Kits Market, Home Safety Test Kits Market Trends, Home Safety T …
“Home Safety Test Kits Market” 2020-2025 Research Report is a professional and in-depth study on the current state of the market. Global Home Safety Test Kits market containing a complete view of the market size, business share, profit estimates, SWOT analysis and the regional landscape of the Industry. The report explains key challenges and future development prospects of the market. The Global Home Safety Test Kits analysis is provided for…
Test Data Management (TDM) Market - test data profiling, test data planning, tes …
The report categorizes the global Test Data Management (TDM) market by top players/brands, region, type, end user, market status, competition landscape, market share, growth rate, future trends, market drivers, opportunities and challenges, sales channels and distributors.
This report studies the global market size of Test Data Management (TDM) in key regions like North America, Europe, Asia Pacific, Central & South America and Middle East & Africa, focuses on the consumption…
Hearing Screening and Diagnostic Devices Market Demands with Major Tests: pure T …
New Market Research Reports Title "Hearing Screening And Diagnostic Devices Market 2018" Has Been Added to Crystal Market Research Report database.
Hearing Screening and Diagnostic Devices - Competitive Insights:
The leading players in the market are Gn Otometrics A/S, Otodynamics, Nashua Hearing Group, Siemens Healthineers, Natus Medical Incorporated, Interacoustics A/S, Neurosoft S.A, Accent Hearing Pty Ltd, MAICO Diagnostics GmbH and IntriCon Corporation. The major players in the market are profiled in detail…
