Press release
Software as a Medical Device (SaMD) Market to Reach US$ 28.5 Billion by 2031 at a CAGR of 69.31%; North America Leads with 44% Share - Key Players: Siemens Healthineers, Philips, Arterys
The global Software as a Medical Device (SaMD) market is experiencing rapid expansion and is estimated to grow at a CAGR of 69.31 percent during the forecast period 2024-2031. The market was valued at approximately US$ 1.02 billion in 2023 and is projected to reach around US$ 28.5 billion by 2031, driven by accelerating digital transformation in healthcare, increasing adoption of AI-driven clinical decision tools, and rising demand for remote monitoring and virtual care solutions.Software as a Medical Device, as defined by the International Medical Device Regulators Forum (IMDRF), refers to software intended for one or more medical purposes that performs these functions independently of a physical medical device. SaMD solutions support various clinical applications, including diagnostics, disease management, imaging analysis, and therapeutic guidance. Their ability to deliver real-time insights, enhance clinical accuracy, and operate across connected ecosystems positions SaMD as a transformative force within modern healthcare. As regulatory frameworks evolve and digital health adoption intensifies, the SaMD market is expected to witness significant scaling across key regions worldwide.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/software-as-a-medical-devices-market?sai-v
The Software as a Medical Device (SaMD) market refers to the industry focused on standalone software solutions that perform medical functions, such as diagnosis, monitoring, or treatment, without being part of a physical medical device.
Key Developments
✅ October 2025: Major U.S. digital health companies expanded AI-driven SaMD platforms for diagnostics and remote disease monitoring, receiving multiple FDA clearances for autonomous decision-support algorithms.
✅ September 2025: European regulatory bodies accelerated approvals under the MDR framework for cloud-based SaMD solutions in cardiology, neurology, and radiology, boosting digital therapeutic adoption.
✅ August 2025: Asia-Pacific healthcare providers integrated SaMD-based clinical decision tools into hospital IT systems to improve workflow efficiency, patient triage, and chronic disease management.
✅ July 2025: Global medical device manufacturers launched next-generation cybersecurity-enhanced SaMD solutions to meet updated safety and interoperability standards for connected health systems.
✅ May 2025: Digital therapeutics and AI-health startups introduced personalized SaMD platforms combining remote monitoring, predictive analytics, and patient engagement modules.
✅ March 2025: Middle Eastern hospitals expanded deployment of SaMD-assisted imaging and screening tools to improve early diagnosis for oncology, dermatology, and cardiovascular diseases.
Mergers & Acquisitions
✅ November 2025: A leading U.S. health-tech company acquired a regulatory-approved AI-diagnostics SaMD developer to strengthen its clinical decision-support offerings.
✅ August 2025: A European digital health software provider formed a strategic partnership with a global cloud infrastructure company to co-develop secure, scalable SaMD platforms.
✅ June 2025: An Asia-Pacific medtech firm acquired a machine learning-based SaMD analytics startup to expand its portfolio of AI-enabled monitoring and diagnostic applications.
Key Players
Arterys Inc. | Paragon Biosciences (Qlarity Imaging LLC) | Viz.ai, Inc. | VitalConnect | Apple Inc. | iSchemaView Inc. (RAPID) | IDx Technologies Inc. | MaxQ AI Ltd. | Greenfinch Technology | Siemens Healthineers AG | Koninklijke Philips N.V.
Key Highlights
Arterys Inc. - Holds a 9.8% share, driven by its cloud-native AI imaging platform, strong FDA-cleared solutions, and expanding adoption across radiology departments.
Paragon Biosciences (Qlarity Imaging LLC) - Holds a 7.1% share, supported by its AI-based breast cancer diagnostic tools, enhanced clinical decision support, and growing oncology footprint.
Viz.ai, Inc. - Holds a 12.4% share, fueled by its AI-powered stroke detection and care coordination platform widely deployed in hospitals worldwide.
VitalConnect - Holds a 5.6% share, driven by its AI-enabled wearable biosensors, remote patient monitoring solutions, and strong engagement in digital health.
Apple Inc. - Holds an 11.3% share, supported by its health-focused ecosystem, advanced biometric monitoring features, and strategic investments in medical-grade diagnostics.
iSchemaView Inc. (RAPID) - Holds a 10.2% share, recognized for its RAPID imaging platform for stroke triage, strong clinical validation, and global hospital partnerships.
IDx Technologies Inc. - Holds a 6.4% share, driven by its FDA-approved autonomous AI diagnostic systems and increasing demand in ophthalmology.
MaxQ AI Ltd. - Holds a 4.9% share, supported by its AI-driven acute care imaging solutions enhancing diagnostic accuracy for critical conditions.
Greenfinch Technology - Holds a 3.8% share, fueled by its AI-based healthcare software development capabilities and integration in clinical imaging workflows.
Siemens Healthineers AG - Holds a 15.7% share, driven by its extensive imaging portfolio, advanced AI platforms, and large global customer base.
Koninklijke Philips N.V. - Holds a 12.8% share, supported by its enterprise imaging solutions, AI-enabled diagnostics, and innovations in patient monitoring.
Purchase this report before year-end and unlock an exclusive 30% discount: https://www.datamintelligence.com/buy-now-page?report=software-as-a-medical-devices-market?sai-v
(Purchase 2 or more Reports and get 50% Discount)
Market Drivers
- Increasing adoption of digital health technologies and AI-driven clinical decision tools across healthcare systems.
- Rising demand for remote patient monitoring, chronic disease management, and virtual care solutions.
- Growing regulatory support from FDA, EMA, and other authorities enabling faster approvals for digital therapeutics and diagnostic software.
- Expansion of cloud computing, mobile health apps, and wearable technologies integrating SaMD functionalities.
- Increasing focus on personalized medicine and data-driven treatment pathways requiring advanced software tools.
- Rising healthcare costs driving adoption of scalable, software-based solutions to improve efficiency and outcomes.
- Growing prevalence of chronic diseases and aging populations increasing demand for continuous monitoring solutions.
Industry Developments
- Launch of AI- and machine learning-powered SaMD applications for diagnostics, imaging interpretation, and therapeutic interventions.
- Strategic collaborations between medtech companies, software developers, and healthcare providers to expand SaMD ecosystems.
- Development of cybersecurity-enhanced platforms to ensure safe data exchange and reduce compliance risks.
- Increasing investment in digital therapeutics (DTx) platforms receiving regulatory and reimbursement approvals worldwide.
- Expansion of SaaS-based SaMD models offering scalability, remote updates, and improved patient engagement.
- Rising integration of SaMD with wearables, smartphones, and IoT-enabled health devices for real-time insights.
Regional Insights
North America - 44% share: "Driven by strong regulatory frameworks, rapid digital health adoption, and significant investment in AI-based SaMD solutions."
Europe - 30% share: "Supported by stringent medical device regulations, rising digital therapeutics adoption, and expanding health IT infrastructure."
Asia Pacific - 20% share: "Fueled by growing digital health initiatives, rising smartphone penetration, and increasing investment in AI-driven healthcare tools."
Latin America - 4% share: "Boosted by expanding telehealth adoption and growing healthcare digitalization efforts."
Middle East & Africa - 2% share: "Driven by smart healthcare projects, improving IT infrastructure, and rising acceptance of remote monitoring tools."
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/software-as-a-medical-devices-market?sai-v
Key Segments
➥ By Device Type
Wearable Devices: Includes smartwatches, fitness trackers, biosensors, and health monitoring wearables that enable continuous tracking of vital parameters and remote patient monitoring.
PCs/Laptops: Used for data analysis, clinical documentation, teleconsultations, and integration with healthcare information systems for efficient medical workflows.
Smartphones/Tablets: Mobile-enabled platforms supporting telehealth, remote diagnostics, patient engagement apps, and real-time health data tracking.
➥ By Application
Diagnostic: Digital tools and connected devices used for early disease detection, real-time monitoring, and remote diagnostic evaluations.
Clinical Management: Solutions enabling treatment planning, medication management, patient monitoring, clinical decision support, and workflow optimization.
➥ By Deployment Method
Cloud-Based: Offers scalable storage, remote accessibility, seamless updates, and interoperability across multiple healthcare systems.
On-Premise: Locally hosted infrastructure ensuring strong control over data security, customization, and compliance for healthcare institutions.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Software as a Medical Device (SaMD) Market to Reach US$ 28.5 Billion by 2031 at a CAGR of 69.31%; North America Leads with 44% Share - Key Players: Siemens Healthineers, Philips, Arterys here
News-ID: 4304794 • Views: …
More Releases from DataM intelligence 4 Market Research LLP

Men's Vitality Supplements Market Set for Steady Growth to USD 11.21 Billion by …
The Global Men's Vitality Supplements Market, valued at US$ 6.53 billion in 2025 and projected to reach US$ 11.21 billion by 2033 at a CAGR of 6.99%, is primarily driven by rising health awareness among men and increasing demand for preventive healthcare solutions. Growing concerns related to low testosterone levels, stress-induced fatigue, reduced stamina, and age-related hormonal imbalances are encouraging men to adopt dietary supplements that enhance energy, immunity, and…
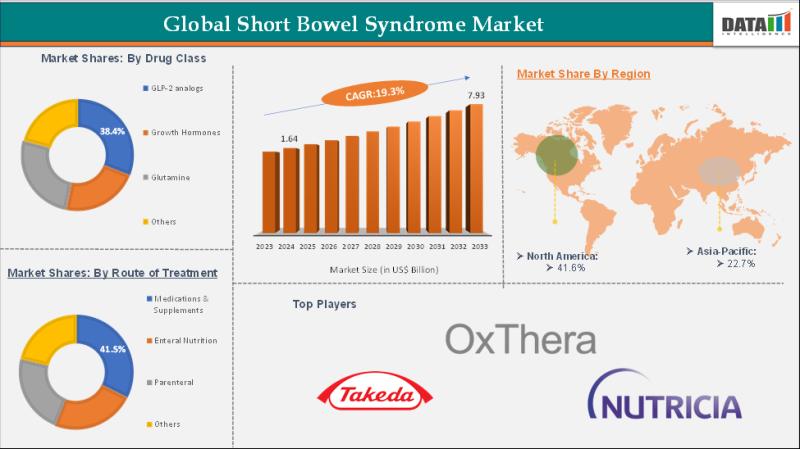
Short Bowel Syndrome Market Set for Explosive Growth to US$ 7.93 Billion by 2033 …
The Global Short Bowel Syndrome Market was valued at US$ 1.64 billion in 2024 and is expected to reach US$ 7.93 billion by 2033, growing at a CAGR of 19.3% during the forecast period 2025-2033.
Market expansion is propelled by the rising prevalence of short bowel syndrome linked to intestinal surgeries, Crohn's disease, and congenital anomalies, alongside increased survival rates from pediatric surgeries. Advancements in GLP-2 analogs like teduglutide, growing adoption…

Dehydrated Vegetables Market Forecast Shows Robust Growth to US$132.3 Billion by …
The Global Dehydrated Vegetables Market reached USD million in 2022 and is projected to witness lucrative growth by reaching up to USD million by 2032. The market is growing at a CAGR of 5.34% during the forecast period (2023-2032).The growth of the global dehydrated vegetables market is primarily driven by the rising demand for convenience and ready-to-eat food products due to changing consumer lifestyles and increasing urbanization. Busy work schedules…

Advanced Driver Assistance Systems Market Set for Explosive Growth to US$165.68 …
The Global Advanced Driver Assistance Systems Market reached US$44.96 billion in 2023 and is expected to reach US$165.68 billion by 2031, growing with a CAGR of 17.71% during the forecast period 2024-2031, according to DataM Intelligence report.
Market growth is propelled by stringent government regulations mandating safety features, rising road accident rates, and rapid advancements in AI, LiDAR, radar, and sensor technologies enabling higher autonomy levels. Increasing consumer demand for enhanced…
More Releases for SaMD
U.S Software as a Medical Device (SaMD) Market Growth & Trends 2025
The US Software as a Medical Device Market reached US$ 205.12 million in 2024 and is expected to reach US$ 715.00 million by 2033, growing at a CAGR of 13.5% during the forecast period 2025-2033.
U.S Software As A Medical Devices Market report, published by DataM Intelligence, provides in-depth insights and analysis on key market trends, growth opportunities, and emerging challenges. Committed to delivering actionable intelligence, DataM Intelligence empowers businesses to…
Software as a Medical Device Market (SaMD): A Game-Changer in Modern Healthcare
Software as a medical devices (SaMD) market is estimated to reach at a CAGR of 69.31% during the forecast period (2024-2031).
Software as a Medical Device (SaMD) refers to standalone software intended for medical purposes without being part of a physical medical device. SaMD can perform tasks such as diagnosing conditions, predicting treatment outcomes, or monitoring chronic diseases. Examples include mobile apps for tracking heart health, AI-driven diagnostic tools, and cloud-based…
Key Influencer in the Software As A Medical Device (SaMD) Market 2025: IOT Revol …
What combination of drivers is leading to accelerated growth in the software as a medical device (samd) market?
The increasing use of the Internet of Things (IoT) within the healthcare industry is likely to fuel the expansion of the software as a medical device (SaMD) market in the future. IoT in healthcare refers to medical equipment and software which can interact with digital networks to gain access to healthcare IT systems.…
Software as a Medical Device (SaMD) Market Size, Share, Trends, and Forecast 202 …
"The Business Research Company recently released a comprehensive report on the Global (SaMD) Software As A Medical Device Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get…
Hottest Trends in 2023 Software as a Medical Device (SaMD) Market| Inzentiz, Jab …
2023-2031 Report on Global Software as a Medical Device (SaMD) Market by Player, Region, Type, Application and Sales Channel is the latest research study released by HTF MI evaluating the market risk side analysis, highlighting opportunities, and leveraging strategic and tactical decision-making support. The report provides information on market trends and development, growth drivers, technologies, and the changing investment structure of the Global Software as a Medical Device (SaMD) Market.…
Software as a Medical Device (SaMD) Market 2021 | Detailed Report
The Software as a Medical Device (SaMD) research report undoubtedly meets the strategic and specific needs of the businesses and companies. The report acts as a perfect window that provides an explanation of market classification, market definition, applications, market trends, and engagement. The competitive landscape is studied here in terms of product range, strategies, and prospects of the market’s key players. Furthermore, the report offers insightful market data and information…
