Press release
Advanced Therapy Medicinal Products (ATMP) Market to Reach USD 61.5 Billion by 2030 at 13.4% CAGR, Led by North America with 45% Share
Global Advanced Therapy Medicinal Products (ATMP) Market was valued at USD 25.5 billion in 2023 and is projected to reach USD 61.5 billion by 2030, growing at a strong CAGR of 13.4% during the forecast period 2023-2030. The market is rapidly gaining momentum as breakthrough therapies transform how chronic, rare, and genetic diseases are treated-shifting from symptom management to true disease modification.ATMP growth is powered by rising adoption of cell therapies, gene therapies, and tissue-engineered products, along with increased prevalence of chronic and inherited disorders. Supportive regulatory pathways, advancements in biotechnology, and expanding investment in personalized and regenerative medicine are further accelerating innovation. These next-generation therapies are redefining the future of healthcare with the potential to restore, repair, or even replace biological functions at their root.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/advanced-therapy-medicinal-product-atmp-market?saikumar
The Advanced Therapy Medicinal Products (ATMP) Market is transforming modern medicine with cutting-edge gene, cell, and tissue therapies designed to treat previously incurable diseases.
Key Developments
✅ October 2025: Hospitals adopted advanced ATMP manufacturing and monitoring systems to support increasing use of gene and cell therapies in clinical care.
✅ July 2025: Bioprocessing companies introduced automated platforms for vector production, improving efficiency and batch reliability.
✅ April 2025: Developers accelerated regulatory submissions for next-generation regenerative therapies, boosting commercialization activity.
✅ September 2025: Allogeneic therapy programs expanded, increasing availability of off-the-shelf regenerative treatments.
✅ June 2025: Regulatory ecosystems strengthened with faster review pathways and incentives for ATMP innovation.
✅ February 2025: Investments surged in tissue-engineered products and gene-editing technologies targeting rare and degenerative diseases.
Mergers & Acquisitions
✅ July 2025: A major ATMP manufacturer acquired a cell-processing technology startup to strengthen production scalability.
✅ March 2025: A pharmaceutical company purchased a gene-editing biotech to expand its regenerative medicine pipeline.
✅ January 2025: A therapeutic developer acquired a viral-vector facility to support late-stage ATMP programs.
✅ April 2025: A European biopharma company partnered with an APAC therapy developer to build next-generation gene and cell therapy platforms.
✅ February 2025: A leading therapy provider acquired a vector-engineering firm to enhance manufacturing capabilities.
✅ January 2025: An international biotech group completed the acquisition of a tissue-engineering specialist to grow its regenerative solutions portfolio.
Key Players
RHEACELL | BioTissue Technologies GmbH | Mukocell GmbH | Orchard Therapeutics plc | Kite Pharma EU B.V. | Bristol-Myers Squibb Pharma EEIG | Novartis Europharm Limited | Amgen Europe B.V.
Key Highlights
• RHEACELL - Holds an estimated 11.4% market share, driven by its strong portfolio in regenerative medicine and advanced cell-based therapeutics targeting rare and chronic conditions.
• BioTissue Technologies GmbH - Accounts for around 7.9% share, supported by its innovative tissue-engineering platforms and expanding applications in reconstructive and regenerative procedures.
• Mukocell GmbH - Maintains approximately 5.6% share, recognized for its urological regenerative therapies and growing demand for minimally invasive tissue-repair solutions.
• Orchard Therapeutics plc - Represents nearly 13.2% share, driven by its leadership in gene therapy for rare diseases and commercial progress across Europe and North America.
• Kite Pharma EU B.V. - Holds an estimated 12.6% share, fueled by its pioneering CAR-T cell therapy portfolio and strong adoption in hematological cancer treatment centers.
• Bristol-Myers Squibb Pharma EEIG - Accounts for around 14.8% share, backed by its extensive pipeline in cell and gene therapy and significant investments in immuno-oncology.
• Novartis Europharm Limited - Maintains approximately 17.5% share, supported by its market-leading cell and gene therapy products, global distribution capabilities, and robust clinical pipeline.
• Amgen Europe B.V. - Represents nearly 16.0% share, driven by its advanced biologics, gene-modulation research, and strong therapeutic presence across oncology, immunology, and rare diseases.
Purchase this report before year-end and unlock an exclusive 30% discount: https://www.datamintelligence.com/buy-now-page?report=advanced-therapy-medicinal-product-atmp-market?saikumar
(Purchase 2 or more reports and get 50% Discount)
Market Drivers
- Increasing prevalence of chronic and rare diseases is accelerating demand for highly targeted and curative treatment approaches.
- Expanding clinical success of gene therapies, cell therapies and tissue-engineered products is improving investor confidence and regulatory support.
- Rising global investment in regenerative medicine infrastructure, bioprocessing technologies and GMP manufacturing facilities is strengthening supply capabilities.
- Technological advancements in viral vectors, cell modification tools and CRISPR platforms are enabling faster development of next-generation ATMPs.
Industrial Developments
- Multiple regulatory agencies advanced fast-track and priority review pathways for novel cell and gene therapies targeting oncology and rare genetic disorders.
- Biopharmaceutical companies expanded their vector manufacturing capacities to overcome bottlenecks in gene therapy production.
- Strategic collaborations between CDMOs and ATMP developers accelerated scale-up and commercialization of autologous and allogeneic cell therapies.
- Growing adoption of AI-driven analytics to optimize cell purity, potency prediction and regulatory documentation processes.
Regional Insights
North America holds over 45% market share due to "rapid adoption of cell and gene therapies, strong reimbursement landscape and advanced biomanufacturing capacity."
Europe accounts for above 30% of the market, supported by "structured regulatory frameworks, strong clinical trial activity and expanding ATMP innovation hubs."
Asia-Pacific is growing at over 15% CAGR, driven by "increasing investments in regenerative medicine, fast-developing biotech ecosystems and rising approval of locally manufactured ATMPs."
Latin America and the Middle East & Africa collectively contribute below 10%, influenced by "gradual improvements in healthcare infrastructure and rising participation in early-stage ATMP research."
Key Segments
➥ By Type
Gene therapy medicines work by modifying or replacing faulty genes to treat or prevent disease, offering long-term or potentially curative outcomes for genetically driven conditions. Somatic cell therapy medicines use manipulated cells-often stem cells or immune cells-to repair, regenerate, or replace damaged tissues, playing a vital role in regenerative medicine and advanced therapeutic treatments. Tissue-engineered medicines combine scaffolds, cells, and biologically active molecules to restore, replace, or enhance tissue function, supporting applications in wound healing, organ repair, and structural regeneration.
➥ By Disease Indication
Cancer remains one of the largest treatment areas, with cell and gene therapies offering targeted, personalized, and highly effective interventions such as CAR-T and tumor-specific gene editing. Cardiovascular diseases benefit from regenerative therapies aimed at repairing heart tissue, improving vascular function, and reducing long-term complications. Musculoskeletal disorders leverage cell-based and tissue-engineered solutions for cartilage repair, bone regeneration, and treatment of degenerative conditions. Immune system and inflammation-related diseases are addressed through therapies that modulate immune responses, restore balance, or correct underlying immune defects. Neurology applications include treatments for genetic neurological disorders, neurodegenerative diseases, and injuries where advanced biologics enable nerve repair or functional restoration. Others include metabolic, ophthalmic, and rare diseases where advanced therapies provide new treatment pathways.
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/advanced-therapy-medicinal-product-atmp-market?saikumar
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Advanced Therapy Medicinal Products (ATMP) Market to Reach USD 61.5 Billion by 2030 at 13.4% CAGR, Led by North America with 45% Share here
News-ID: 4299753 • Views: …
More Releases from DataM intelligence 4 Market Research LLP

Artificial Intelligence (AI) Chip Market (2026-2033) | UAE's 4 Trillion Transist …
DataM Intelligence has published a new research report on "Artificial Intelligence (AI) Chip Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Latest M & A
• Intel moves…

Japan Nurse Call Systems Market (2025-2033) | Opportunities in Hospitals and Sen …
DataM Intelligence has published a new research report on "Japan Nurse Call Systems Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Get a Sample PDF Of This…
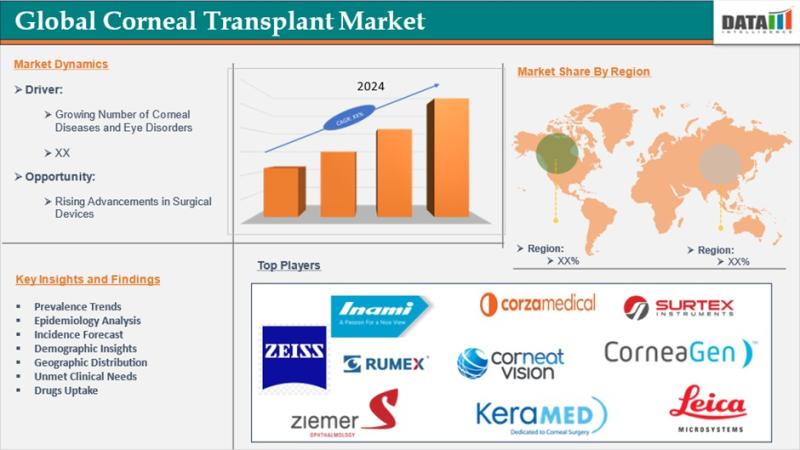
Corneal Transplant Market to Reach US$ 1,006.95 Million by 2033 at 7.59% CAGR | …
Corneal Transplant Market reached US$ 523.88 million in 2024 and is expected to reach US$ 1,006.95 million by 2033, growing at a CAGR of 7.59% during the forecast period 2025 to 2033.
Corneal transplantation, also known as keratoplasty, is a surgical procedure in which a damaged or diseased cornea is replaced with healthy donor tissue to restore vision and maintain ocular integrity. The cornea serves as the transparent outer layer of…
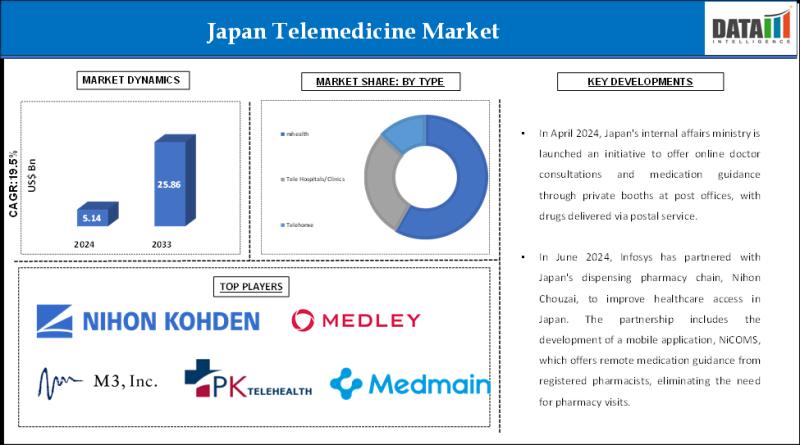
Japan Telemedicine Industry (2025-2033) | Technology Advancements and Market Pen …
DataM Intelligence has published a new research report on "Japan Telemedicine Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Get a Sample PDF Of This Report (Get…
More Releases for ATMP
Amino Trimethylene Phosphonic Acid (ATMP) Market Trends, Challenges and Strategi …
According to QY Research announces the release of 2025 latest report "Amino Trimethylene Phosphonic Acid (ATMP) Market". Based on current situation and impact historical analysis (2020-2024) and forecast calculations (2025-2031), this report provides a comprehensive analysis of the global Amino Trimethylene Phosphonic Acid (ATMP) market, including market size, share, demand, industry development status, and forecasts for the next few years.
Download Exclusive PDF Sample: (Including Full TOC, Data Tables, Visual Charts)…
ATMP Service Providers Market Driven by Rising Adoption of Personalized Medicine …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global ATMP Service Providers Market by Type of Services (Analytics/CRO (Microbiology, Endotoxin, PCR, Flow Cytometry, ELISA, Container Closure, Sterilty, and Pre-clinical), Quality (Documentation, QMS And Regulations), CMO (HQ/GMP plasmid DNA, MSC Manufacture, Pluripotent Stem Cells), Logistics (Storage, Stability And Transport)), Application (GTMP(Gene Therapy Medicinal Products), sCTMP(somatic Cells Therapy Medicinal Products) and TEP(Tissue Engineered Products))-Trends, Industry Competition…
Lambda Biologics Secures Saxony Grant for ATMP Development with ORGANOIDSCIENCES
Image: https://www.getnews.info/uploads/88c7b29c2c2475f99f24c910a7580594.jpg
Lambda Biologics GmbH, located in the Leipzig Bio Cluster in Germany and led by CEO Andre Gerth, operates a biohealth testing platform. The company is growing as both a testing platform and a hub for knowledge and research, focusing not only on accurate test results but also on streamlining and advancing researchers' work processes.
Lambda Biologics received a public grant of the Free State of Saxony (Germany) in October to…
Advanced Therapy Medicinal Products (ATMP) Service Providers Market Exclusive Ov …
Advanced Therapy Medicinal Products (ATMP) Service Providers Market worth $34.59 Billion by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global ATMP Service Providers Market by Type of Services (Analytics/CRO (Microbiology, Endotoxin, PCR, Flow Cytometry, ELISA, Container Closure, Sterilty, and Pre-clinical), Quality (Documentation, QMS And Regulations), CMO (HQ/GMP plasmid DNA, MSC Manufacture, Pluripotent Stem Cells), Logistics (Storage, Stability…
Advanced Therapy Medicinal Products (ATMP) Service Providers Market | Know the L …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global ATMP Service Providers Market by Type of Services (Analytics/CRO (Microbiology, Endotoxin, PCR, Flow Cytometry, ELISA, Container Closure, Sterilty, and Pre-clinical), Quality (Documentation, QMS And Regulations), CMO (HQ/GMP plasmid DNA, MSC Manufacture, Pluripotent Stem Cells), Logistics (Storage, Stability And Transport)), Application (GTMP(Gene Therapy Medicinal Products), sCTMP(somatic Cells Therapy Medicinal Products) and TEP(Tissue Engineered Products))-Trends, Industry Competition…
ATMP Production Cost Analysis Report, Raw Materials Requirements, Costs and Key …
The latest report titled "ATMP Production Cost Report" by Procurement Resource, a global procurement research and consulting firm, provides an in-depth cost analysis ofa the production process of the ATMP. Read More: https://www.procurementresource.com/production-cost-report-store/atmp
Report Features - Details
Product Name - ATMP
Process Included - ATMP Production From Phosphorous Acid
Segments Covered
Manufacturing Process: Process Flow, Material Flow, Material Balance
Raw Material and Product/s Specifications: Raw Material Consumption, Product and Co-Product Generation, Capital Investment
Land and Site Cost:…
