Press release
Neupril Reaches One Million Customers With US-Manufactured Methylfolate Supplements
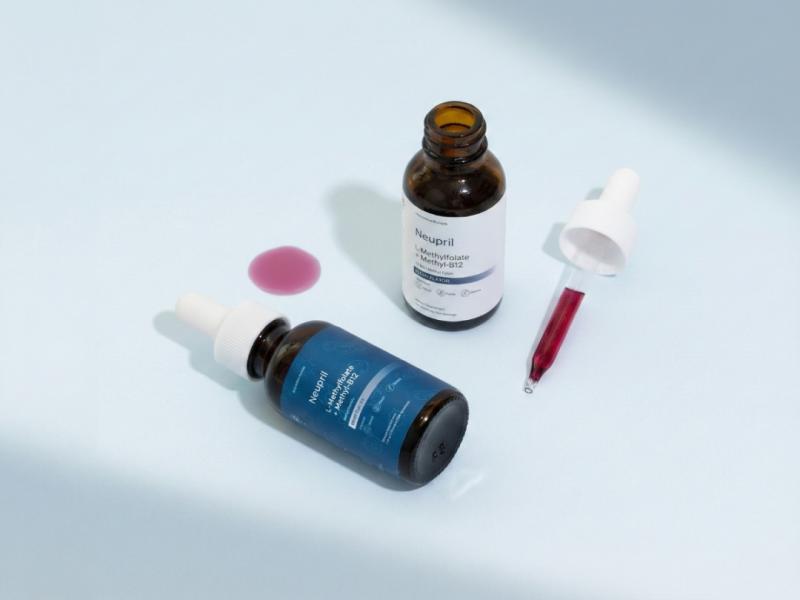
Neupril Pure Methylfolate -- Manufactured in the USA in an FDA-registered, GMP-certified facility and third-party tested for purity and potency.
American-Made Supplement Company Reinforces Commitment to Domestic Production and Independent Third-Party Testing
Neupril, a health and wellness company specializing in methylfolate supplementation, has announced reaching the milestone of one million customers served while maintaining its commitment to United States-based manufacturing and comprehensive third-party quality testing.
The company produces all of its liquid L-methylfolate supplements domestically in an FDA-registered, GMP-certified manufacturing facility located in the United States. This commitment to American manufacturing ensures products meet rigorous federal quality and safety standards enforced by U.S. regulatory authorities.
Quality Assurance Through Third-Party Testing
Central to Neupril's operations is a comprehensive third-party testing protocol. Every batch of Neupril supplements undergoes independent laboratory analysis before distribution to consumers. This third-party testing includes potency verification to confirm label accuracy, heavy metals screening for lead, mercury, arsenic, and cadmium, and microbial contamination testing to ensure product safety.
The company's manufacturing facility maintains Current Good Manufacturing Practice (cGMP) certification as required by the U.S. Food and Drug Administration for supplement production. This FDA-registered facility undergoes regular compliance audits and inspections to maintain its certification status.
Domestic Operations and Fulfillment
All Neupril products are manufactured in the United States and shipped from American-based fulfillment centers. Domestic customers typically receive orders within three to seven business days through standard U.S. shipping carriers, with tracking information provided for all shipments.
The company maintains customer support operations and offers a 30-day satisfaction guarantee on all purchases, allowing customers to return products if not satisfied.
Product Formulation
Neupril's flagship adult formula provides 15 milligrams of L-5-Methylfolate calcium salt combined with Methylcobalamin, the active form of Vitamin B12. The liquid drop format is designed for efficient absorption and ease of use.
The company also manufactures Neupril Kids Pure Methylfolate, a pediatric formula with age-appropriate dosing for children ages one through eighteen. Both products are produced in the same FDA-registered, GMP-certified, and third-party tested facility.
All Neupril formulations are non-GMO, vegan-friendly, and free from gluten, dairy, soy, nuts, artificial colors, and artificial preservatives.
Customer Response and Company Growth
Neupril reports a 4.7-star average rating based on over 45,000 customer reviews. The company attributes its growth to consistent product quality, transparent manufacturing practices, and responsive customer service.
About Methylfolate Supplementation
L-Methylfolate, also designated as 5-MTHF, represents the biologically active form of folate that circulates in the human bloodstream. Research indicates that genetic variations in the MTHFR enzyme affect folate metabolism in a significant percentage of the population. Unlike synthetic folic acid, L-methylfolate does not require enzymatic conversion and is immediately available for biological processes.
About Neupril
Neupril is a United States-based health and wellness company focused on methylfolate supplementation. The company manufactures all products in an FDA-registered, GMP-certified American facility, with every batch verified through independent third-party laboratory testing. Neupril serves customers nationwide through its website at neupril.com [https://neupril.com/].
For more information, visit neupril.com [https://neupril.com/]
Media Contact
Company Name: Neupril
Contact Person: John Riley
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=neupril-reaches-one-million-customers-with-usmanufactured-methylfolate-supplements]
Phone: 3072155697
Address:5830 east 2nd street suite 7000
City: Casper
State: WY
Country: United States
Website: http://www.neupril.com
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Neupril Reaches One Million Customers With US-Manufactured Methylfolate Supplements here
News-ID: 4295258 • Views: …
More Releases from ABNewswire

ECI Jewelers Introduces Streamlined Selling Experience with Same-Day Offers and …
ECI Jewelers has enhanced its luxury watch and jewelry buying service with same-day market-based offers and instant payment options for sellers nationwide. The concierge-style approach includes free valuations, full insurance coverage during transit, and expert assessment from the company's New York City Diamond District location, simplifying the selling process for owners of premium timepieces from brands like Rolex, Patek Philippe, and Audemars Piguet.
Elegant Creations Inc, operating as ECI Jewelers, has…

DivorceGO Simplifies the Uncontested Divorce Process Across Ontario
DivorceGO Family Law Offices is helping Ontario couples move forward with clarity and efficiency through its streamlined uncontested divorce services.
DivorceGO Family Law Offices is helping Ontario couples move forward with clarity and efficiency through its streamlined uncontested divorce services. Designed for spouses who agree on ending their marriage, the firm's approach focuses on minimizing conflict, reducing stress and guiding clients through the legal process with transparency and fixed-fee pricing.
An uncontested…

Half a Century of Roots: Antelope Valley Honors Tip Top Arborists with "Best of …
Tip Top Arborists, Lancaster's longest-standing tree care company, has been nominated for the Antelope Valley Press Best of 2026 awards as it marks 50 years in business. Founded in 1976, the TCIA-accredited company employs ISA-Certified Arborists, holds a 4.9-star rating, and provides true 24/7 emergency service. Community members can vote at avpress.com/bestof2026 through March 3, 2026.
LANCASTER, Calif. - February 27, 2026 - In a rapidly changing world, few things remain…

Marco Robinson Launches Public 'Proof of Work' Portal to Enable Independent Veri …
Primary-source documentation published to support independent review by media, partners and the public
LONDON - Feb. 27, 2026 - British entrepreneur, author and media producer Marco Robinson today opened a public Proof of Work portal containing primary-source documentation intended to enable independent verification of his business activities, media projects and legal record.
The portal, which is published on Robinson's official website, presents source documents rather than commentary, including solicitor correspondence, court outcomes…