Press release
Global Viral Clearance Service Market to Reach USD 2.8 Bn by 2031, Driven by Surge in Biologics Production and Intensifying Regulatory Demands
The global Viral Clearance Service Market is poised for substantial expansion over the next decade, supported by the rapid rise in biologics manufacturing, increased investment in biosafety testing, and tightening regulatory frameworks governing pharmaceutical development. According to industry analysis, the market valued at USD 857.4 Mn in 2022 is projected to grow at a robust CAGR of 14.6% from 2023 to 2031, reaching USD 2.8 Bn by 2031.Introduction: Viral clearance services have emerged as a crucial component of the biopharmaceutical development ecosystem. From early-stage drug discovery to large-scale biologics manufacturing, these services ensure that drugs, vaccines, and therapeutic proteins remain free from viral contamination. As pharmaceutical companies accelerate their focus on advanced biologics-including vaccines, monoclonal antibodies, cell therapies, and recombinant proteins-the need for reliable viral clearance methods has surged to unprecedented levels.
Access important conclusions and data points from our Report in this sample - https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=53502
Market Overview: The Viral Clearance Service Market encompasses a diverse portfolio of services designed to identify, remove, and inactivate harmful viral contaminants across the drug development lifecycle. These services are utilized extensively in clinical phase I-III trials, biologics manufacturing, downstream purification processes, and regulatory validation studies.
A key role of viral clearance services is to demonstrate the capability of purification systems to eliminate adventitious viruses-an essential requirement mandated by global regulatory authorities such as the U.S. FDA, EMA, and several Asian regulatory bodies. Increasing demand for biologic therapies, rising clinical trial complexity, and higher expectations for viral safety standards continue to shape the market's evolution.
Key Drivers of Market Growth
1. Rise in Investment in Biological Testing
Rapid growth in biologics-including cell-based therapies and blood-derived products-has increased the risk of viral contamination during production. Since many biologics originate from human or animal cell cultures, stringent viral safety assessments are not optional but mandated. Regulatory bodies require pharmaceutical manufacturers to prove that their production lines have the capability to remove and inactivate viruses.
This has resulted in a surge in investment toward biosafety laboratories, detection technologies, and high-performance viral testing equipment. Companies such as Merck KGaA have expanded their biologics testing capacity globally, including new laboratory spaces to support large-scale viral clearance studies.
2. Surge in Production of Biologics
The biologics industry is witnessing exponential growth. As highlighted during CPHI 2023, global demand for biologics is expected to rise from 2,500 KL in 2022 to 4,400 KL in 2027-an annual growth rate of more than 11%. This rapid expansion has markedly increased the need for dependable viral safety programs to ensure patient protection and regulatory compliance.
Biopharmaceutical manufacturers rely heavily on viral clearance partners to manage the increased workload and technical complexity associated with large-scale biologics production.
Latest Market Trends
Several key trends are shaping the future of the viral clearance services landscape:
• Advanced Virus Inactivation Technologies
Vendors are introducing innovative virus inactivation reagents and processes. In November 2023, Merck launched its Deviron detergent portfolio, designed to exceed the performance of conventional inactivation methods and meet evolving regulatory standards.
• Growing Outsourcing to Specialized Service Providers
Pharmaceutical companies are increasingly partnering with third-party biosafety laboratories to access state-of-the-art technology without the need for heavy infrastructure investment.
• Rising Adoption of GLP/GMP-Compliant Services
Regulatory bodies now require rigorous documentation and standardized testing protocols, increasing demand for GLP-grade and GMP-certified viral clearance testing.
• Strategic Global Expansion of Testing Facilities
Key players like Texcell, Eurofins, and WuXi AppTec are building or expanding facilities in Asia, Europe, and North America to meet rising global demand.
Key Players and Industry Leaders
Prominent companies dominating the Viral Clearance Service Market include:
• Texcell
• Eurofins Scientific SE
• Charles River Laboratories International, Inc.
• Merck KGaA
• WuXi AppTec
• Clean Cells
• Vironova Biosafety AB
These companies offer high-quality viral removal, inactivation, and validation services, leveraging advanced detection technologies and deep regulatory expertise.
Recent Developments
Recent strategic developments include:
• Summit Pharmaceuticals - Texcell Japan Partnership (Dec 2023)
Summit Pharmaceuticals International signed an exclusive distributorship deal with Texcell Japan to expand access to GLP/GMP-certified biosafety and viral clearance solutions.
• Merck's Launch of Deviron Detergent (Nov 2023)
Merck introduced a new virus inactivation detergent portfolio to help biopharmaceutical companies meet updated regulatory demands more efficiently.
• Texcell Facility Expansion (2023)
Texcell expanded manufacturing capacity in China and the U.S., reinforcing its global leadership in viral clearance and biosafety testing.
Market Opportunities and Challenges
Opportunities
• Growing biologics pipeline: More than 1,000 biologics are currently in development globally.
• Rise in outsourcing: Small and mid-sized biopharma firms increasingly rely on specialized viral clearance vendors.
• Expansion in emerging markets: Asia Pacific is becoming a hotspot for biologics manufacturing and biosafety services.
• Technological advancements: AI-enabled virus detection and high-throughput purification systems offer new possibilities.
Challenges
• High testing costs: Viral clearance studies involve expensive reagents, biosafety-level facilities, and highly trained personnel.
• Evolving regulatory landscape: Companies must remain compliant with stricter global standards.
• Complexity of biologics: Recombinant proteins, gene therapies, and vaccines require highly customized viral clearance protocols.
Future Outlook
From 2023 to 2031, the viral clearance market will expand at 14.6% CAGR, driven by a strong biopharmaceutical pipeline and rising global investments in biologics manufacturing. North America will remain the dominant region due to the presence of major manufacturers, advanced biosafety infrastructure, and robust regulatory enforcement by agencies like the FDA.
Asia Pacific, especially China, India, and Japan, is expected to witness the fastest growth, supported by capacity expansion, cost-effective testing capabilities, and a thriving biologics market.
Advancements in virus removal technologies-including advanced filtration membranes and novel detergent formulations-will further accelerate market progress.
Buy this Premium Research Report and secure exclusive access to insights - https://www.transparencymarketresearch.com/checkout.php?rep_id=53502<ype=S
Market Segmentation
By Method
• Viral Removal
• Viral Inactivation
o Chemical
o Radiation
o Others
By Application
• Recombinant Proteins
• Tissue and Blood-derived Products
• Vaccines
• Others
By End-User
• Biopharmaceuticals
• Contract Research Organizations (CROs)
• Academic Research Institutes
• Others
By Region
• North America (U.S., Canada)
• Europe (Germany, U.K., France, Italy, Spain)
• Asia Pacific (China, Japan, India, Australia & New Zealand)
• Latin America (Brazil, Mexico)
• Middle East & Africa (GCC countries, South Africa)
Why Buy This Report?
• Comprehensive Market Insights: Includes detailed data on market size, forecasts, and CAGR through 2031.
• Deep Competitive Intelligence: Profiles of leading companies, their strategies, recent developments, and financial performance.
• Regulatory Analysis: Offers clarity on global viral safety standards and compliance frameworks.
• Opportunity Mapping: Identifies high-growth segments and emerging opportunities across regions.
• Strategic Guidance for Investors: Helps stakeholders assess risks, devise investment plans, and capitalize on the biological manufacturing boom.
• Detailed Segmentation: Provides granular analysis across method, application, end-user, and regional categories.
• Value-Chain Analysis: Enables understanding of supplier dynamics and end-to-end viral clearance workflows.
Explore Latest Research Reports by Transparency Market Research:
Anti-Infective Drugs Market: https://www.transparencymarketresearch.com/antiinfective-drugs-market.html
Erectile Dysfunction (ED) Drugs Market: https://www.transparencymarketresearch.com/erectile-dysfunction-drugs.html
Pain Management Therapeutics Market: https://www.transparencymarketresearch.com/pain-management-therapeutics.html
Psoriasis Treatment Market: https://www.transparencymarketresearch.com/psoriasis-treatment-market.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact Us:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Viral Clearance Service Market to Reach USD 2.8 Bn by 2031, Driven by Surge in Biologics Production and Intensifying Regulatory Demands here
News-ID: 4289892 • Views: …
More Releases from Transparency Market Research
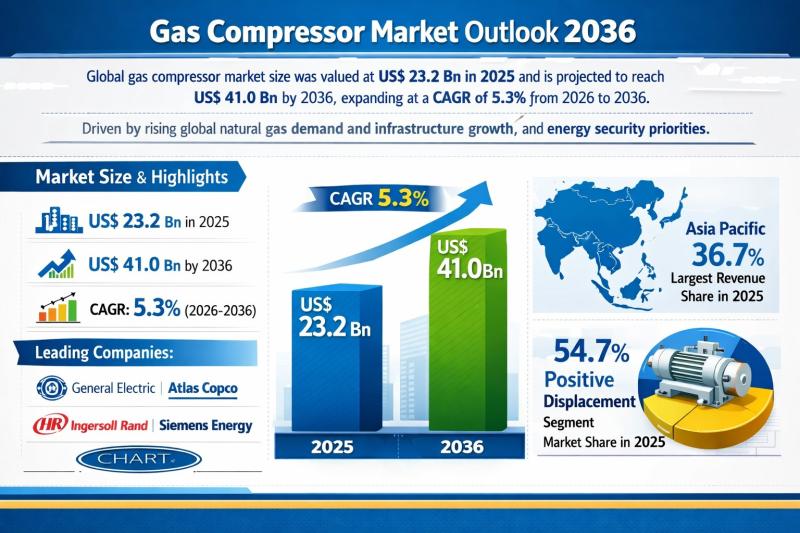
Gas Compressor Market Outlook 2036: Global Industry Expected to Reach US$ 41.0 B …
The global gas compressor market was valued at US$ 23.2 Bn in 2025 and is projected to reach US$ 41.0 Bn by 2036, expanding at a compound annual growth rate (CAGR) of 5.3% from 2026 to 2036. This steady growth trajectory reflects the structural importance of gas compression systems across upstream, midstream, and downstream gas value chains. Rising natural gas consumption, expansion of pipeline and LNG infrastructure, and national energy…
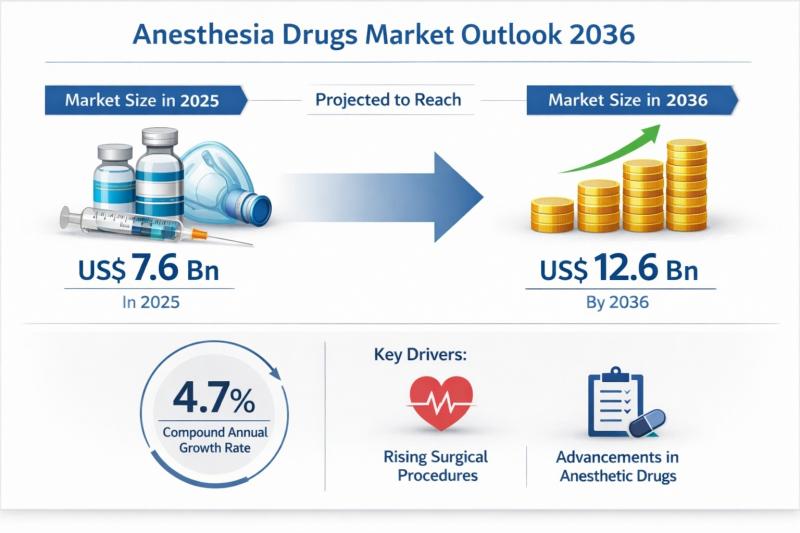
Anesthesia Drugs Market to be Worth USD 12.6 Bn by 2036 - By Drug / By Applicati …
The global anesthesia drugs market was valued at US$ 7.6 billion in 2025 and is projected to reach US$ 12.6 billion by 2036, expanding at a compound annual growth rate (CAGR) of 4.7% from 2026 to 2036. This steady growth trajectory reflects the essential and non-substitutable role of anesthesia drugs in modern healthcare systems. As surgical interventions continue to rise globally-across both elective and emergency procedures-the demand for safe, effective,…
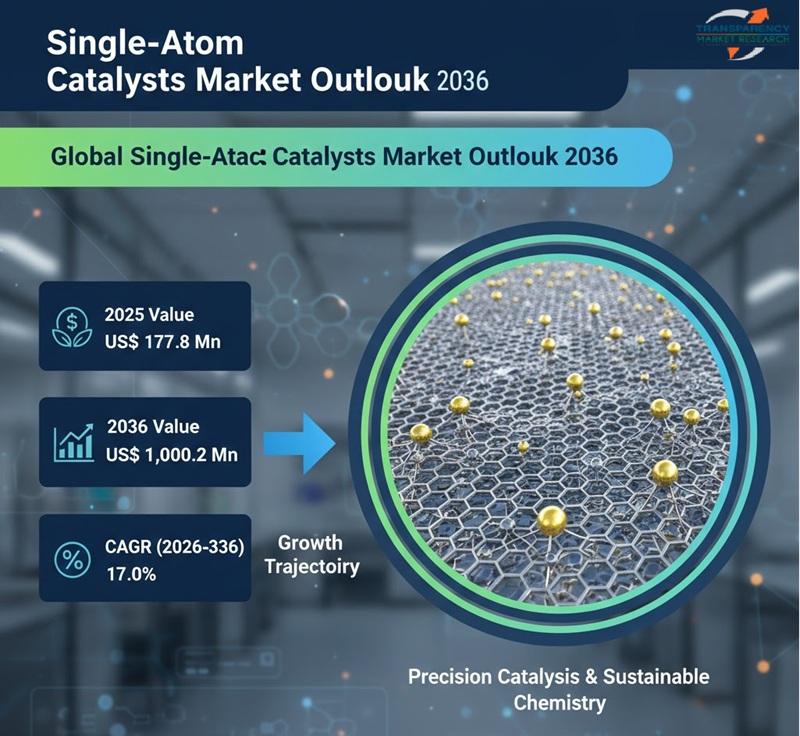
Single-Atom Catalysts Market Size is Expected to Expand from US$ 177.8 Million t …
The global single-atom catalysts (SACs) market is poised for remarkable growth as industries seek highly efficient, cost-effective, and sustainable catalytic solutions. Valued at US$ 177.8 million in 2025, the market is projected to reach US$ 1,000.2 million by 2036, expanding at a robust compound annual growth rate (CAGR) of 17.0% from 2026 to 2036. This rapid expansion reflects the growing importance of advanced catalysis in energy, chemicals, environmental protection, and…
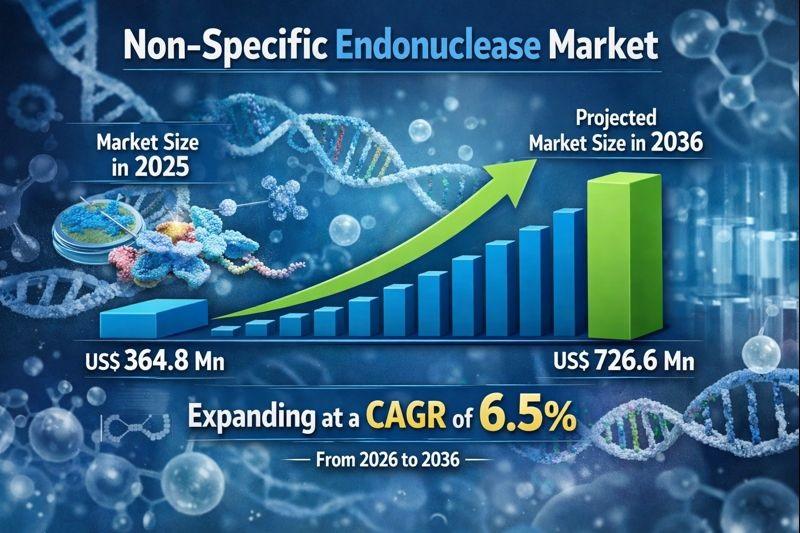
Non-specific Endonuclease Market to Reach USD 726.6 Million by 2036, Supported b …
The non-specific endonuclease market is witnessing steady growth, driven by the expanding use of molecular biology tools across biotechnology, pharmaceuticals, diagnostics, and academic research. Non-specific endonucleases are enzymes that cleave nucleic acids without requiring a specific recognition sequence, making them highly valuable for applications such as DNA/RNA degradation, sample preparation, viscosity reduction, and contamination control. Their broad activity profile differentiates them from restriction enzymes and enables versatile usage across multiple…
More Releases for Viral
Viral and Non Viral Vector Manufacturing Market Share Research Report 2025
On Mar 20, 2025, Global Info Research released a research report titled "Global Viral and Non Viral Vector Manufacturing Market 2025 by Manufacturers, Regions, Type and Application, Forecast to 2031". This report provides detailed data analysis of the Viral and Non Viral Vector Manufacturing market from 2020 to 2031. Including the market size and development trends of Viral and Non Viral Vector Manufacturing Market, it analyzes market size indicators such…
Viral Inactivation Market Report 2024 - Viral Inactivation Market Size, Trends A …
"The Business Research Company recently released a comprehensive report on the Global Viral Inactivation Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
According to The Business Research Company's, The viral inactivation market size…
Viral Filtration Market - Unleashing the Power of Filtration: Safeguarding again …
Newark, New Castle, USA: The "Viral Filtration Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Viral Filtration Market: https://www.growthplusreports.com/report/viral-filtration-market/8028
This latest report researches the industry structure, sales, revenue,…
Viral Clearance Market - Unleash the Power of Clearance: Unrivaled Viral Protect …
Newark, New Castle, USA: The "Viral Clearance Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Viral Clearance Market: https://www.growthplusreports.com/report/viral-clearance-market/7823
This latest report researches the industry structure, sales, revenue,…
Viral Traffic Code - Is The Viral Traffic Code LEGIT?
Viral Traffic Code is a digital program that offers specific strategies to make affiliate earnings making use of a various technique stream. Read this review to get more information about the Viral Website Traffic Code!
Official Web Site: Go Here - https://www.glitco.com/get-Viral-Traffic-Code
What is Viral Website Traffic Code?
Viral Website Traffic Code is a basic system that might aid you make associate profits from various trusted internet sites, including Amazon, ebay.com, Shopify, ClickBank,…
Viral Inactivation Market Growing Demand of Kits and Reagents Viral Inactivation …
According to Precision Business Insights (PBI), the latest report, the Viral Inactivation market is expected to be worth USD 2.1 billion in 2022, growing at a 12.8% CAGR from 2022 to 2028. The primary driver of the expansion of the global Viral Inactivation market are speedy growth in pharmaceutical and biotechnology industries and strong inclination of R&D investments in life sciences industry.
View the detailed report description here - https://precisionbusinessinsights.com/market-reports/viral-inactivation-market/
Kits &…
