Press release
Viral Vectors for Non-human Primates Market to Reach More than USD 429.9 Million by 2034, Expanding at a CAGR of 10.5% | Transparency Market Research
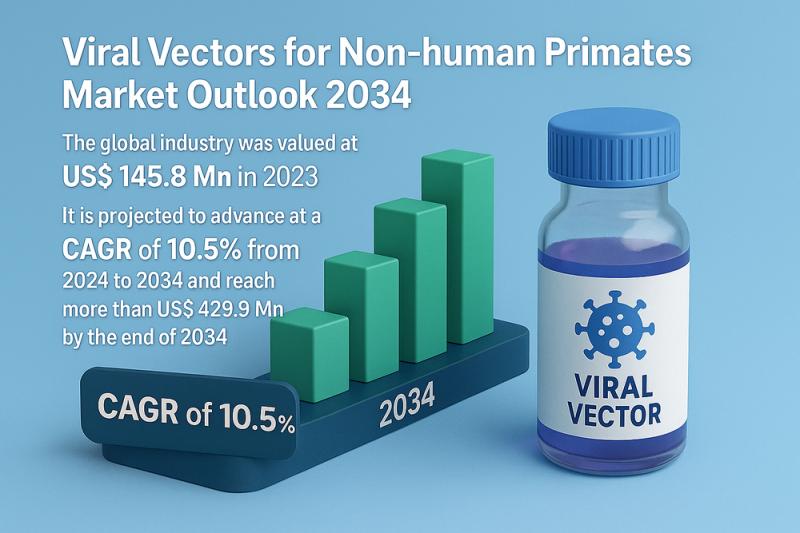
The global Viral Vectors for Non-human Primates Market is witnessing significant momentum driven by the rapid expansion of gene therapy, gene-modified cell therapies, vaccine research, and regenerative medicine. As non-human primates (NHPs) remain one of the most critical preclinical research models due to their genetic and physiological similarity to humans, the demand for highly efficient and safe viral vectors customized for NHP studies continues to rise.According to the latest market assessment, the global industry, valued at US$ 145.8 million in 2023, is projected to expand at a CAGR of 10.5% between 2024 and 2034, reaching more than US$ 429.9 million by 2034. Continuous innovation in vector engineering, a growing clinical pipeline, and an increase in preclinical studies are expected to shape the future of this dynamic market.
Discover valuable insights and findings from our Report in this sample - https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=86344
Market Overview: Viral vectors have emerged as indispensable tools in biomedical research and therapeutic development. These vectors are engineered viruses designed to deliver genetic material into target cells. Their broad utility spans gene therapy, gene-modified cell therapies, vaccine research, oncolytic therapies, and diagnostic applications.
For non-human primates-such as marmosets, rhesus macaques, cynomolgus monkeys, and other species-viral vectors are essential for evaluating gene expression, safety, and efficacy before human trials. Their biological closeness to humans makes NHP research a critical step in translational science.
The market includes major biotechnology companies, academic research centers, and emerging biotech startups. Established players benefit from robust manufacturing infrastructure, whereas startups continue to introduce breakthrough innovations in vector optimization and delivery mechanisms.
Key Drivers of Market Growth
1. Advancements in Gene Therapies and Gene-Modified Cell Therapies
Gene therapy is experiencing unprecedented growth. In 2023, 10% of all innovative approvals by the U.S. FDA were gene and cell therapies, marking a clear acceleration compared to previous years. A rising number of advanced therapies-such as the first CRISPR-based treatment approved in the U.S.-is fueling this momentum.
Currently:
• 35 gene and gene-modified therapies are in Phase III clinical trials
• 282 therapies are in Phase II
• 301 therapies are in Phase I
• 1471 therapies remain in preclinical development
CAR-T cell therapies account for 52% of the gene-modified therapy pipeline, followed by TCR-NK, CAR-M, and TAC-T therapies.
As the preclinical stages of these therapies heavily rely on NHP models to validate efficacy, biodistribution, and safety, demand for viral vectors tailored for these animals is surging.
2. Expanding Therapeutic Applications of Viral Vectors
Viral vectors-especially AAV, lentiviral, adenoviral, and HSV vectors-enable precise gene delivery and controlled gene expression. Initially applied to rare genetic disorders, viral-vector-based treatments are now expanding into more common diseases, including oncology, ophthalmology, neurology, infectious diseases, and metabolic disorders.
As of 2022:
• 120 viral-vector therapies were in Phase II trials
• 25 therapies were under late-stage development
The growing scope of gene editing and gene replacement therapies is widening the applicability of viral vectors, significantly bolstering market growth.
Latest Market Trends
• Shift Toward AAV and Lentiviral Vectors
AAV and lentiviral vectors dominate due to their proven safety, minimal immunogenicity, and long-term gene expression capabilities.
• Rising Use of Viral Vectors in Vaccine Research
The success of viral-vector-based vaccines has accelerated the use of similar platforms for infectious diseases and cancer immunotherapies.
• Rapid Scale-Up of Manufacturing Capabilities
Companies are expanding GMP manufacturing, analytical testing, and bioprocess development to address rising global demand.
• AI and Automation in Vector Engineering
AI-driven optimization, high-throughput screening, and automated vector design are enhancing precision and reducing development timelines.
Key Players and Industry Leaders
The market is characterized by a mix of global biotechnology leaders and rapidly expanding innovators. Prominent companies include:
• Lonza
• Thermo Fisher Scientific Inc.
• Revvity
• VectorBuilder Inc.
• Creative Biolabs
• Takara Bio Inc.
• Merck KGaA
• Andelyn Biosciences
• Biovian Oy
• Genezen
• Others Prominent Players
These companies are intensifying investment in R&D, manufacturing capacity, and strategic partnerships to strengthen market competitiveness.
Recent Developments
• Merck KGaA Acquires Mirus Bio (May 2024)
Merck announced a US$ 600 million acquisition of Mirus Bio, a developer of cutting-edge transfection reagents critical for viral vector production.
• Takara Bio Partners with Gap Junction Therapeutics (June 2023)
The companies joined forces to co-develop AAV vector gene therapy targeting GJB2 gene mutations linked to hearing loss.
• Lonza Expands CGT Development Laboratories (October 2022)
Lonza announced expansions at its U.S. and Netherlands facilities, strengthening global capabilities in viral vector analytical development.
These developments reflect a strong industry push toward scaling viral vector manufacturing, improving technological efficiency, and accelerating therapeutic development.
Market New Opportunities and Challenges
Opportunities
• Growing R&D in Neuroscience, Oncology, and Rare Diseases
Expanding pipelines offer strong opportunities for NHP viral vector suppliers.
• Increasing Government and Private Funding
Global investments in CGT R&D continue to rise sharply.
• Emergence of Next-Generation Vectors
Innovations like synthetic AAV capsids, self-inactivating vectors, and enhanced tropism vectors open new commercial avenues.
• Expansion into Emerging Markets
Asia Pacific countries, especially China, India, and Japan, are expanding preclinical research infrastructure.
Challenges
• High Manufacturing Costs
Producing GMP-grade viral vectors remains costly and time-intensive.
• Complex Regulatory Pathways
Ensuring safety, biodistribution, and quality requires stringent compliance.
• Limited Availability of NHP Models
Ethical concerns, strict regulations, and shortages in certain regions may hinder research timelines.
• Scalability Limitations
Startups often struggle to scale production without strategic partnerships.
Future Outlook
The next decade is expected to witness transformative growth in the viral vectors for non-human primates market. Continuous advancements in gene therapies, along with rising adoption of NHP models in preclinical studies, will significantly influence market expansion.
Key future expectations include:
• Scaling Manufacturing Platforms to meet rising demand for clinical-grade and preclinical-grade vectors
• Advanced, highly targeted vector designs, including synthetic and engineered capsids
• Greater global collaborations across academia, biotech firms, and pharmaceutical companies
• Enhanced focus on safety, immunogenicity reduction, and long-term efficacy
By 2034, the market is expected to surpass US$ 429.9 million, driven by scientific innovation and surging research output.
Buy this Premium Research Report for exclusive, in-depth insights - https://www.transparencymarketresearch.com/checkout.php?rep_id=86344<ype=S
Market Segmentation
By Vector Type
• Adenoviral Vectors
• Adeno-associated Vectors
• Retroviral Vectors
• Lentiviral Vectors
• Other Vectors (including Baculoviral)
By Type of Non-Human Primates
• Marmosets
• Rhesus Macaques
• Cynomolgus Monkeys
• Others (Capuchin Monkeys, Chimpanzee, etc.)
By Application
• Gene Therapy
• Vaccine Research
By Therapeutic Area
• Genetic Disorders
• Infectious Diseases
• Oncological Disorders
• Others
By End User
• Pharmaceutical and Biotechnology Companies
• Academic and Research Institutions
By Region
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East & Africa
North America currently dominates with 48.3% share in 2023, driven by advanced biotechnology ecosystems, strong clinical pipelines, and major research institutions.
Why Buy This Report?
• Comprehensive analysis of market size, growth projections, and segment forecasts
• Detailed review of drivers, trends, challenges, and opportunities
• Extensive competitive landscape, including company profiles and strategic developments
• Insightful Porter's Five Forces, value chain, and regulatory landscape
• Deep-dive analysis into vector technologies, therapeutic pipelines, and NHP-based research
• Coverage across 20+ countries, offering robust regional insights
Explore Latest Research Reports by Transparency Market Research:
Medical Transcription Services Market: https://www.transparencymarketresearch.com/medical-transcription-services.html
Medical Education Market: https://www.transparencymarketresearch.com/medical-education-market-report.html
Operating Room Management Market: https://www.transparencymarketresearch.com/operating-room-management-market.html
Revenue Cycle Management Market: https://www.transparencymarketresearch.com/revenue-cycle-management-market-report.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact Us:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Viral Vectors for Non-human Primates Market to Reach More than USD 429.9 Million by 2034, Expanding at a CAGR of 10.5% | Transparency Market Research here
News-ID: 4289886 • Views: …
More Releases from Transparency Market Research
Wall Putty Market Outlook 2036: Global Industry Set to Cross US$ 1.34 Billion by …
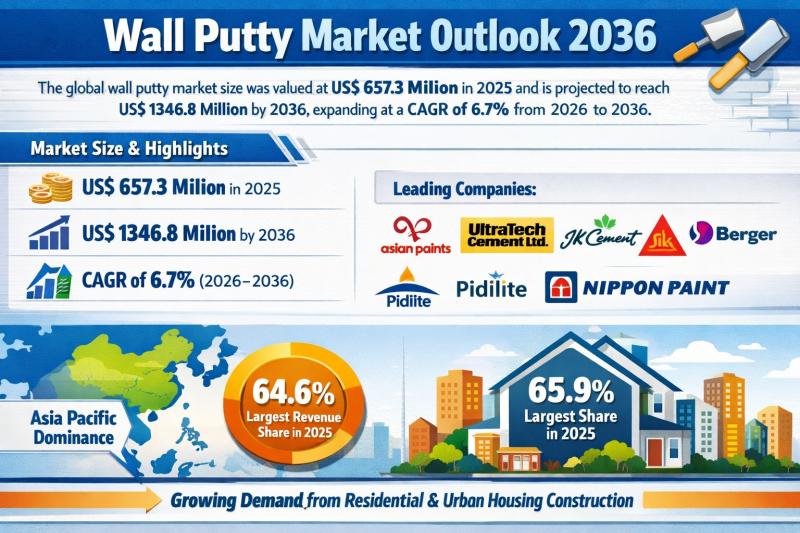
The global wall putty market was valued at US$ 657.3 Million in 2025 and is forecast to reach US$ 1,346.8 Million by 2036, expanding at a steady CAGR of 6.7% from 2026 to 2036. This near-doubling of market value over the forecast period reflects the rising standardization of wall finishing materials in construction specifications and the growing importance of surface preparation for long-lasting paint finishes.
With residential construction activity accelerating across…
Battery Recycling Market Expanding at 10.14% CAGR Through 2036 - By Battery Type …
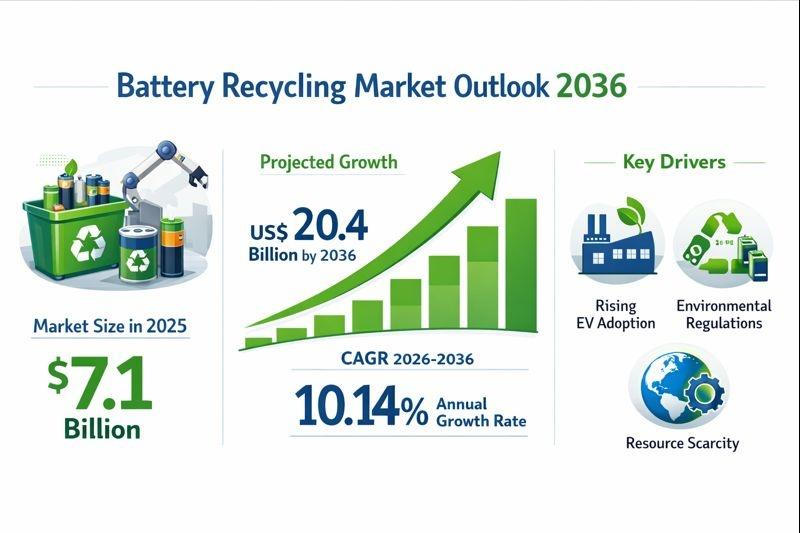
The global Battery Recycling Market was valued at US$ 7.1 billion in 2025 and is projected to reach US$ 20.4 billion by 2036, expanding at a robust compound annual growth rate (CAGR) of 10.14% during the forecast period from 2026 to 2036.
Get a concise overview of key insights from our Report in this sample -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=9061
This substantial growth trajectory reflects the rapid expansion of electric vehicles (EVs), renewable energy storage systems,…
Fiber-Based Blister Pack Market Set to Surge from US$ 1,633.8 Mn to US$ 13,591.8 …
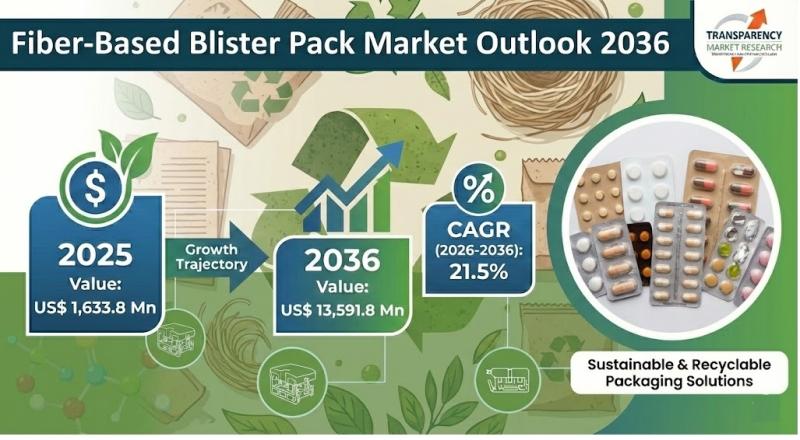
The global fiber-based blister pack market is entering a phase of rapid transformation as sustainability becomes a central priority in packaging across pharmaceuticals, consumer goods, and food applications. Valued at US$ 1,633.8 million in 2025, the market is projected to reach an impressive US$ 13,591.8 million by 2036, expanding at a strong CAGR of 21.5% from 2026 to 2036. This exceptional growth trajectory highlights the accelerating shift away from plastic-based…

Hydroponics Substrate Market Valued at USD 2,467.6 Million, Driven by Controlled …
The global hydroponics substrate market was valued at USD 760.0 million in 2025 and is projected to reach USD 2,467.6 million by 2036, expanding at a compound annual growth rate (CAGR) of around 11.3% during the forecast period. This growth is driven by the rapid adoption of hydroponic farming, vertical agriculture, and controlled environment cultivation across commercial and urban farming setups. Rising demand for high-yield crops, efficient water utilization, and…
More Releases for Vector
Growing Prevalence Of Vector-Borne Diseases Fuels Vector Control Market: Pivotal …
Stay ahead with our updated market reports featuring the latest on tariffs, trade flows, and supply chain transformations.
Vector Control Market Size Growth Forecast: What to Expect by 2025?
The market size of vector control has been experiencing robust growth in the past years, and it's predicted to increase from $20.53 billion in 2024 to $22.01 billion in 2025, with a compound annual growth rate (CAGR) of 7.2%. Factors such as urbanization…
Global Viral Vector Manufacturing Market Size by Application, Type, and Geograph …
USA, New Jersey- According to Market Research Intellect, the global Viral Vector Manufacturing market in the Internet, Communication and Technology category is projected to witness significant growth from 2025 to 2032. Market dynamics, technological advancements, and evolving consumer demand are expected to drive expansion during this period.
The viral vector manufacturing market is witnessing accelerated growth due to the rising demand for gene therapies and vaccines. These vectors are essential tools…
Prominent Viral Vector Manufacturing Market Trend for 2025: Product Innovation I …
How Are the key drivers contributing to the expansion of the viral vector manufacturing market?
The escalation in the occurrence of both cancer and contagious diseases is anticipated to spur the expansion of the viral vector manufacturing market in the future. An infectious disease is a condition caused by a virus or its harmful byproduct which spreads to a susceptible organism through contact with an infected individual, creature, or object. Cancer…
Emerging Lentiviral Vector Market Trend 2025-2034: Technological Advancements In …
How Is the Lentiviral Vector Market Projected to Grow, and What Is Its Market Size?
The lentiviral vector market will grow from $14.37 billion in 2024 to $16.62 billion in 2025, at a CAGR of 15.7%. This market is expanding due to the growing prevalence of genetic disorders, increasing demand for gene delivery systems, biotech and pharmaceutical investments, and regulatory approvals for related therapies.
The lentiviral vector market is expected to grow…
Viral Vector Manufacturing Market Report 2024 - Viral Vector Manufacturing Marke …
"The Business Research Company recently released a comprehensive report on the Global Viral Vector Manufacturing Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive…
Adeno-Associated Virus Vector Manufacturing Market - Building Better Therapies: …
Newark, New Castle, USA - Growth Plus Reports has published a new report on Adeno-Associated Virus Vector Manufacturing Market, which includes a detailed analysis based on competitors and important market segments (2023-2031). The Global Adeno-Associated Virus Vector Manufacturing provides a thorough analysis of many segments such as opportunities, market size, developments, innovation, sales, and overall growth of leading players. The research is based on primary and secondary statistical data, and…