Press release
Pharmacogenomics Testing Center Business Plan 2025 : Investment Requirements and Market Growth Outlook
Pharmacogenomics Testing Center Business Plan & Project Report OverviewIMARC Group's "Pharmacogenomics Testing Center Business Plan and Project Report 2025" offers a comprehensive framework for establishing a successful pharmacogenomics testing center business. The critical areas, including market trends, investment opportunities, revenue models, and financial forecasts, are discussed in this in-depth report and are therefore useful resources to entrepreneurs, consultants and investors. Whether evaluating the viability of a new venture or streamlining an existing one, the report gives an in-depth analysis of all the ingredients that make it successful, starting with business formation and profitability over time.
What is a Pharmacogenomics Testing Center Business?
A Pharmacogenomics Testing Center is a medical practice which uses genetic testing to provide genomic-based precision medicine, medication management, DNA analysis, drug-gene interaction testing and evidence-based medication algorithm recommendations to health care providers and their patients, in order to assist in optimizing pharmaceutical treatment outcomes, and prescribing in accordance with a patient's genetic profile.
Services offered include pharmacogenetic testing panels, thorough DNA tests, medication compatibility tests, drug metabolism tests, tests for risk of adverse reaction to drug therapy, personalized dosing recommendations, genetic counseling, clinical consultations, and continual medication management programs that help patients get safer and more effective pharmaceutical therapy.
They include clinical pharmacogenomics labs, precision medicine centers, genetic testing labs, clinics with personalized medication management, and clinics with precision medicine services that offer accurate genetic sequencing, clinically relevant testing, appropriate evidence-based interpretation, provider collaboration, patient education, treatment optimization assistance, continuing medical education, and clinical engagement with patients and providers.
The Pharmacogenomics Testing Centers have developed state-of-the-art laboratory equipment, DNA sequencing technology, advanced bioinformatics tools, genetic data analysis systems and clinical decision making and evidence-based medicine software applications. They have also developed secure patient data management systems, integrated healthcare technology, and precision medicine analytics.
Depending upon context, they may be specialized clinical pharmacogenomics laboratories, precision medicine centers, integrated healthcare testing centers, or full personalized medication optimization centers delivering complete genetically guided pharmaceutical care experiences to various segments of the population with varying levels of medication complexity and varied therapeutic requirements.
Request for a Sample Report: https://www.imarcgroup.com/pharmacogenomics-testing-center-business-plan-project-report/requestsample
Pharmacogenomics Testing Center Business Market Trends and Growth Drivers
The trends and underlying drivers of growth in the market for a Pharmacogenomics Testing Center business include the increased use of precision medicine in outpatient settings, the increased awareness of adverse drug reactions or ineffectiveness of a drug, the increased hospitalizations because of medications, and the increased adoption of personalized medicine through genetic profiling. Potential drivers for clinical genetics services include the rise of precision medicine, evidence-based pharmaceutical therapy guidelines, genetic-informed guidelines and precision medication management, the preference of healthcare providers for validated genetic tests, optimized treatment options, and convenient access to actionable genetic information in the emerging precision healthcare ecosystem.
In order to meet these needs, operators are investing in advanced genetic testing platforms, clinical validation networks, evidence-based testing environments, education platforms for healthcare providers, and clinical laboratory accreditation processes to improve the patient and healthcare provider experience and create positive business outcomes in a world trending towards precision medicine and personalized healthcare.
Other revenue sources may include clinical consultation services, healthcare providers, partnerships with the pharmaceutical industry, medication management and adherence programs, genetic counseling, continuing medical education programs, research participation agreements with patients, and the commercialization of genetic testing services.
Important factors about a center's location include its presence in local health care systems, specialty medical practices, network of hospital systems, and referring physicians, as these can provide a steady referral base and impart a level of credibility to the center. State-of-the-art laboratories, compliance with clinical laboratory standards of operation, and adherence to genetic testing certification requirements help ensure quality operations and build trust with healthcare providers.
Threats to the business include rapid advancements in genetic testing capabilities which may render current testing methods obsolete, competition from existing clinical laboratories and national testing companies, dependency on reimbursement policies of the healthcare system and insurance companies, and regulatory restrictions on laboratory certification requirements and genetic testing certification rules.
Pharmacogenomics Testing Centers have a business model that requires capital investment for the clinical laboratory and precision medicine facilities, the genetic and bioinformatics testing equipment, the reagents inventory, the laboratory analysts, the genetic counselors, and effective marketing and education of clinical use, local healthcare, and planned physician, healthcare systems and specialty medical practices partnerships. By providing high-quality genetic tests and clinically actionable results and an outstanding experience for healthcare providers, they can increase patient safety and encourage healthcare providers to adopt evidence-based personalized medication management solutions for their patients rather than prescribing medications based on trial and error.
Report Coverage
The Pharmacogenomics Testing Center Business Plan and Project Report includes the following areas of focus:
• Business Model & Operations Plan
• Technical Feasibility
• Financial Feasibility
• Market Analysis
• Marketing & Sales Strategy
• Risk Assessment & Mitigation
• Licensing & Certification Requirements
The comprehensive nature of this report ensures that all aspects of the business are covered, from market trends and risk mitigation to regulatory requirements and precision medicine-focused healthcare provider acquisition strategies.
Key Elements of Pharmacogenomics Testing Center Business Setup
Business Model & Operations Plan
A solid business model is crucial to a successful venture. The report covers:
• Service Overview: A breakdown of pharmacogenetic testing procedures, DNA sample processing, genetic analysis protocols, drug-gene interaction assessments, clinical consultation services, genetic counseling programs, medication optimization support, and healthcare provider collaboration services offered
• Service Workflow: How each patient sample collection, laboratory processing, genetic analysis, clinical interpretation, healthcare provider reporting, genetic counseling, and clinical follow-up process is managed
• Revenue Model: An exploration of the mechanisms driving revenue across multiple testing categories and clinical consultation services
• SOPs & Service Standards: Guidelines for consistent testing quality, clinical validation standards, laboratory accreditation practices, and patient care satisfaction
This section ensures that all operational and clinical aspects are clearly defined, making it easier to scale and maintain service quality.
Buy Report Now: https://www.imarcgroup.com/checkout?id=38833&method=1911
Technical Feasibility
Setting up a successful business requires proper laboratory and clinical infrastructure planning. The report includes:
• Location Selection Criteria: Key factors to consider when choosing laboratory locations and target healthcare markets
• Space & Costs: Estimations for required laboratory space, clinical consultation areas, genetic counseling rooms, and associated costs
• Equipment & Systems: Identifying essential genetic sequencing equipment, DNA extraction systems, bioinformatics platforms, and clinical decision support technology
• Laboratory & Clinical Setup: Guidelines for creating advanced pharmacogenomic testing facilities and evidence-based clinical consultation areas
• Utility Requirements & Costs: Understanding the specialized laboratory infrastructure and utilities necessary to run genetic testing operations
• Human Resources & Wages: Estimating staffing needs, roles, and compensation for laboratory technicians, genetic counselors, clinical consultants, bioinformaticians, and support personnel
This section provides practical, actionable insights into the laboratory and clinical infrastructure needed for setting up your business, ensuring testing accuracy and clinical excellence.
Financial Feasibility
The Pharmacogenomics Testing Center Business Plan and Project Report provides a detailed analysis of the financial landscape, including:
• Capital Investments & Operating Costs: Breakdown of initial and ongoing investments
• Revenue & Expenditure Projections: Projected income and cost estimates for the first five years
• Profit & Loss Analysis: A clear picture of expected financial outcomes
• Taxation & Depreciation: Understanding tax obligations and equipment depreciation
• ROI, NPV & Sensitivity Analysis: Comprehensive financial evaluations to assess profitability
This in-depth financial analysis supports effective decision-making and helps secure funding, making it an essential tool for evaluating the business's potential.
Market Insights & Strategy
Market Analysis
A deep dive into the pharmacogenomics testing center market, including:
• Industry Trends & Segmentation: Identifying emerging trends and key market segments across clinical pharmacogenomics laboratories, precision medicine centers, genetic testing facilities, medication management clinics, and healthcare-integrated testing establishments
• Regional Demand & Cost Structure: Regional variations in pharmacogenomic testing adoption and cost factors affecting laboratory operations
• Competitive Landscape: An analysis of the competitive environment including established national testing laboratories, regional genetic testing companies, hospital-based pharmacogenomics programs, and precision medicine-focused facilities
Profiles of Key Players
The report provides detailed profiles of leading players in the industry, offering a valuable benchmark for new businesses. It highlights their strategies, testing offerings, clinical programs, and market positioning, helping you identify strategic opportunities and areas for differentiation.
Capital & Operational Expenditure Breakdown
The report includes a comprehensive breakdown of both capital and operational costs, helping you plan for financial success. The detailed estimates for facility development, equipment, and operating costs ensure you're well-prepared for both initial investments and ongoing expenses.
• Capital Expenditure (CapEx): Focused on laboratory space renovation and design, genetic sequencing equipment, bioinformatics systems, clinical consultation furniture, genetic counseling areas, and clinical decision support technology
• Operational Expenditure (OpEx): Covers ongoing costs like staff salaries, genetic testing reagent costs, utilities, marketing expenses, accreditation fees, clinical validation costs, and facility maintenance
Financial projections ensure you're prepared for cost fluctuations, including adjustments for genetic testing technology updates, reimbursement policy changes, accreditation renewal costs, and competitive market pressures over time.
Profitability Projections
The report outlines a detailed profitability analysis over the first five years of operations, including projections for:
• Total revenue from genetic testing services, clinical consultations, and healthcare partnerships, expenditure breakdown, gross profit, and net profit
• Profit margins for each revenue stream and year of operation
• Revenue per test projections and market penetration growth estimates
These projections offer a clear picture of the expected financial performance and profitability of the business, allowing for better planning and informed decision-making.
Request For Customization: https://www.imarcgroup.com/request?type=report&id=38833&flag=E
Our expertise includes:
• Market Entry and Expansion Strategy
• Feasibility Studies and Business Planning
• Company Incorporation and Healthcare Laboratory Setup Support
• Regulatory and Licensing Navigation
• Competitive Analysis and Benchmarking
• Industry Partnership Development
• Branding, Marketing, and Precision Medicine-Focused Provider Strategy
About Us
IMARC Group is a leading global market research and management consulting firm. We specialize in helping organizations identify opportunities, mitigate risks, and create impactful business strategies.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: (+1-201971-6302)
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pharmacogenomics Testing Center Business Plan 2025 : Investment Requirements and Market Growth Outlook here
News-ID: 4289205 • Views: …
More Releases from IMARC Group

Indonesia Logistics Market Expected to Reach USD 132.2 Billion by 2034, Industry …
IMARC Group's latest research publication "Indonesia Logistics Market Size, Share, Trends and Forecast by Model Type, Transportation Mode, End Use, and Region, 2026-2034" the Indonesia logistics market size reached USD 72.4 Billion in 2025. The market is expected to reach USD 132.2 Billion by 2034, exhibiting a growth rate (CAGR) of 6.91% during 2026-2034.
Request a Sample Report: https://www.imarcgroup.com/indonesia-logistics-market/requestsample
What is Logistics?
Logistics refers to the comprehensive process of planning, implementing, and controlling…
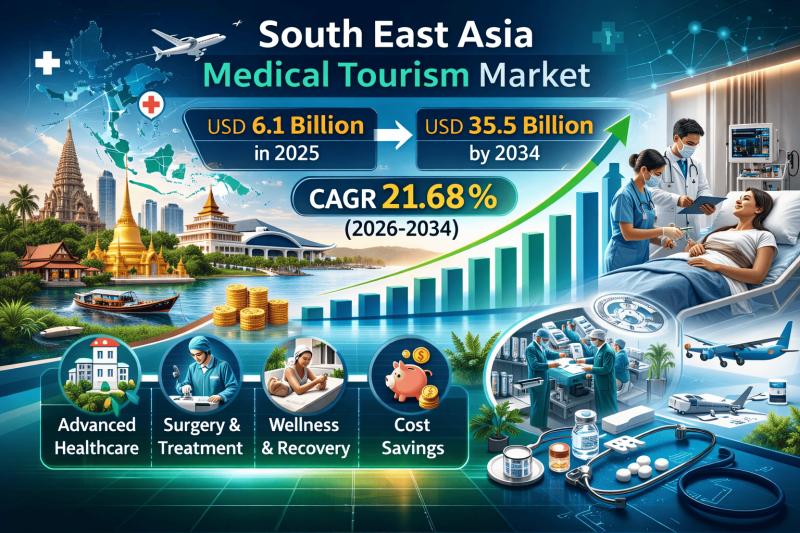
South East Asia Medical Tourism Market Expected to Reach USD 35.5 Billion by 203 …
IMARC Group's latest research publication "South East Asia Medical Tourism Market Size, Share, Trends and Forecast by Treatment Type, Service Provider, and Country, 2026-2034" the South East Asia medical tourism market size reached USD 6.1 Billion in 2025. The market is expected to reach USD 35.5 Billion by 2034, exhibiting a growth rate (CAGR) of 21.68% during 2026-2034.
Request a Sample Report: https://www.imarcgroup.com/south-east-asia-medical-tourism-market/requestsample
What is Medical Tourism?
Medical tourism refers to the practice…

Bamboo Plywood Manufacturing Plant Cost 2026: Detailed Project Report & Profit A …
Setting up a Bamboo Plywood Manufacturing Plant positions investors in a growing and sustainable segment of the engineered wood and construction materials industry, backed by sustained global demand driven by eco-conscious construction, renewable material adoption, and increasing requirements for sustainable furniture and interior solutions. As urbanization accelerates and demand for environmentally friendly building products rises, bamboo plywood offers dual benefits: reducing reliance on traditional hardwoods while providing a strong, durable,…

Paper Cups Manufacturing Plant Cost 2026: CapEx, OpEx & ROI Analysis
Setting up a Paper Cups Manufacturing Plant positions investors in one of the most stable and essential segments of the sustainable packaging value chain, backed by sustained global growth driven by rapid expansion of foodservice and beverage industry, rising consumption of takeaway beverages, increasing restrictions on plastic-based disposables, and the hygienic, eco-friendly, lightweight advantages of paper cup packaging. As café culture accelerates globally, regulatory frameworks increasingly ban single-use plastics, and…
More Releases for Pharmacogenomics
Pharmacogenomics - Top Companies Regional and Data Analysis
Pharmacogenomics is a branch of science, which studies the role of gene over the activity of a drugs and hence helps the refreshers in predicting the response of novel as well as previously available therapies.
Get More Details with Sample PDF Copy @ https://www.theinsightpartners.com/sample/TIPRE00007564/?utm_source=OpenPR&utm_medium=10129
Major Companies operating in the Pharmacogenomics Market are:
• F. Hoffmann-la Roche Ltd
• Abbott
• Oxford Nanopore Technologies
• Thermo Fisher Scientific Inc.
• Illumina, Inc.
• QIAGEN
• Agilent Technologies, Inc.
• Myriad Genetics, Inc.
• Admera Health
Contact Us:
If you have any queries about…
Pharmacogenomics Market worth $5.8 billion by 2028
North America region is witnessing increasing investments and research activities in the field of drug discovery and development. The need for advanced therapies due to rising rate of cancer has contributed to growth of the pharmacogenomics market.
Pharmacogenomics Market [https://www.marketsandmarkets.com/Market-Reports/pharmacogenomics-market-142682251.html?utm_source=ABnewswire&utm_campaign=Paid&utm_content=Referral] in terms of revenue was estimated to be worth $3.5 billion in 2023 and is poised to reach $5.8 billion by 2028, growing at a CAGR of 10.6% from 2023 to…
Genetic Medicine: Unveiling the Power of Pharmacogenomics
Based on technology, the pharmacogenomics market is segmented into PCR, sequencing, microarray, gel electrophoresis, mass spectrometry, and others. The market for the PCR segment is subsegmented into real time PCR, standard PCR, and digital PCR. The PCR segment dominated the pharmacogenomics market in 2021 and is expected to register the highest CAGR during the forecast period.
Download Sample PDF Details at: https://www.theinsightpartners.com/sample/TIPRE00007564?utm_source=OpenPR&utm_medium=10776
The pharmacogenomics market is segmented on the basis of…
Complete PDF Guide On Pharmacogenomics [PDF]
According to our latest study on "Pharmacogenoics Market Forecast to 2028 - COVID-19 Impact and Global Analysis - by Technology, Application, and End User," the market is projected to reach US$ 14,107.80 million by 2028 from US$ 7,087.81 million in 2021; it is expected to grow at a CAGR of 10.3% from 2021 to 2028. The report highlights the key factors driving the market growth and prominent players with their…
Global Pharmacogenomics Market Size & Trends
According to a new market research report published by Global Market Estimates, the global pharmacogenomics market is projected to grow at a CAGR of 9.6% from 2023 to 2028.
The growth of the global pharmacogenomics market is driven by advancements in genomics and DNA sequencing technologies, the rising demand for personalized medicine, regulatory support from agencies like the FDA, cost-effectiveness through reduced adverse reactions, and a growing aging population with complex…
Global Pharmacogenomics Services Market Insights | 2022-2032
The Pharmacogenomics Services Market refers to the services provided by companies and laboratories that offer personalized medicine based on a patient's genetic makeup. Pharmacogenomics is the study of how an individual's genes affect their response to certain drugs, and the pharmacogenomics services market focuses on utilizing this knowledge to optimize drug therapy.
These services typically involve genetic testing to identify specific genetic variations that may impact a patient's response to certain…
