Press release
Clinical Trial Supplies Market Set to Double by 2035 Amid Rising Global R&D Investments
The Clinical Trial Supplies Market is undergoing a major transformation as biopharmaceutical companies accelerate drug development, adopt decentralized trial models, and expand global research footprints. Valued at USD 4.6 billion in 2025, the market is projected to reach USD 9.7 billion by 2035, growing at a strong CAGR of 7.8%. This growth reflects the rising complexities of clinical study designs, increasing demand for biologics, and the rapid integration of digital and advanced cold-chain technologies across global research networks.As pharmaceutical sponsors and CROs push toward efficiency, sustainability, and patient-centricity, several aligned segments within this ecosystem-including the Clinical Trial Materials Market and the Clinical Research Supplies Market-are becoming integral to seamless research operations. Together, these markets ensure the reliable manufacturing, packaging, storage, and distribution of investigational products across geographically dispersed trial sites.
In recognition of Black Friday, we are offering a special 15% discount on this comprehensive research report, providing stakeholders with exceptional value and strategic intelligence.
Buy Now: https://marketgenics.co/buy/clinical-trial-supplies-market-01651
Rising Complexity of Trials Fuels Market Expansion
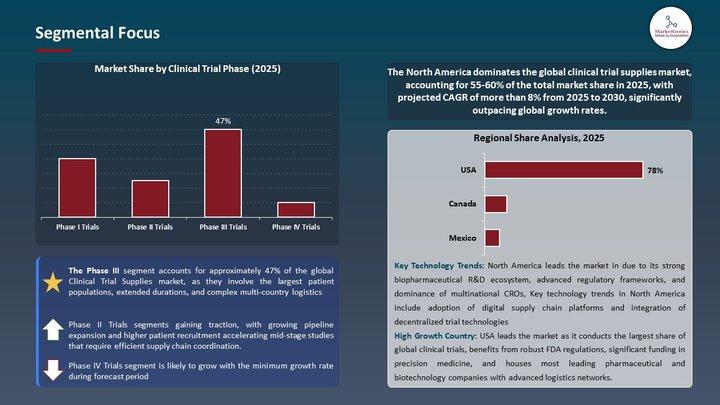
One of the strongest forces shaping the Clinical Trial Supplies Market is the growing complexity of clinical research. Oncology, rare diseases, gene therapies, and personalized medicine require highly specialized supply chain solutions, advanced temperature control systems, and rapid logistics. Phase III trials, which involve the largest patient populations, represent nearly 47% of total market demand in 2025, highlighting the critical role of large-scale coordination and multi-country distribution.
Companies such as Thermo Fisher, Catalent, and Lonza are expanding global capacity to support these complex needs. For instance, Thermo Fisher's expansion of its Kentucky and Pennsylvania facilities in 2024 strengthened its ability to manage oncology and rare disease trial supplies. Similarly, Catalent doubled its European storage and distribution capacity to meet the surging demand for personalized drugs and temperature-sensitive materials.
This increasing supply chain sophistication directly influences not only the Clinical Trial Supplies Market, but also the broader Clinical Trial Materials Market, which relies on precise forecasting, quality control, and compliant manufacturing practices.
Cold Chain Management: A Key Challenge and Opportunity
As the volume of biologics, vaccines, and cell therapies increases, cold chain logistics have become both a critical enabler and a major restraint for the market. Maintaining product integrity across global trial networks requires ultra-low-temperature freezers, specialized packaging, and certified storage infrastructure.
UPS subsidiary Marken has invested heavily in CAR-T cell therapy logistics, expanding its ultra-low-temperature storage sites in 2023 and 2024. However, these systems are expensive to maintain, especially for smaller biotech companies conducting international trials. Failure in cold chain management can result in product loss, compliance penalties, and trial delays-making it a central challenge for stakeholders throughout the Clinical Research Supplies Market.
At the same time, the growing focus on precision medicine and biologics ensures that cold chain innovation is one of the most attractive future opportunities. Companies investing in cryogenic capabilities, sustainability-driven packaging, and IoT-enabled temperature monitoring stand to gain competitive advantage.
Get the Detailed Industry Analysis (including the Table of Contents, List of Figures, and List of Tables) - from the Clinical Trial Supplies Market Research Report: https://marketgenics.co/reports/clinical-trial-supplies-market-01651
Growth of Decentralized and Hybrid Trials Reshaping Supply Models
Decentralized clinical trials (DCTs) are rapidly transforming how investigational products are delivered. Direct-to-patient shipping, home health support, telemedicine-compatible packaging, and real-time inventory tracking are now essential components of modern trial management.
Catalent's launch of decentralized clinical supply services in 2024 marked a major step toward patient-centric research. This approach reduces site visits, improves patient retention, and increases accessibility-especially across rural or underserved regions.
The shift to decentralized research models creates strong growth momentum for both the Clinical Trial Supplies Market and the Clinical Trial Materials Market, as suppliers are required to provide:
Localized storage
Flexible distribution methods
Trackable packaging
Automated temperature monitoring
Patient-specific kit customization
The integration of digital systems ensures accuracy, minimizes wastage, and supports regulatory compliance across multiple countries.
AI and Digitalization Driving a Data-Driven Supply Chain
Artificial intelligence, cloud platforms, and predictive analytics are emerging as pivotal tools within the Clinical Research Supplies Market. These technologies help sponsors and CROs analyze patient recruitment data, forecast material demand, and optimize supply routes.
In March 2024, Parexel partnered with Microsoft Azure to integrate AI-based supply chain forecasting into oncology trials. By predicting material needs in real time, sponsors reduce both overstocking and the risk of product shortages, improving trial efficiency and cost-effectiveness.
AI also supports:
Real-time monitoring of investigational product use
Automated compliance documentation
Risk analysis for trial delays
Smart distribution route planning
Digitalization strengthens the resilience, speed, and agility of the Clinical Trial Supplies Market, especially as multi-regional studies become more common.
To know more about the Clinical Trial Supplies Market - Download our Sample Report: https://marketgenics.co/download-report-sample/clinical-trial-supplies-market-01651
North America Leads Global Market with Strong Infrastructure
North America remains the largest and most advanced market, accounting for 59.7% of global revenue in 2025. The region benefits from:
A strong pharmaceutical and biotechnology presence
High clinical trial activity
Sophisticated regulatory frameworks
Expansive cold chain infrastructure
Early adoption of digital supply technologies
Companies such as Thermo Fisher, UPS, DHL, and Almac continue to make multimillion-dollar investments to strengthen the supply chain backbone supporting global trials.
With its robust ecosystem, North America remains central not only to the Clinical Trial Supplies Market, but also to the connected Clinical Trial Materials Market and Clinical Research Supplies Market.
Key Market Players Strengthening Global Networks
The market is moderately consolidated, with Tier-1 players controlling over 45% of the global industry. Leading companies include:
Thermo Fisher / Fisher Clinical Services
Catalent
Almac Group
PCI Pharma Services
Parexel
Marken (UPS)
Lonza
Strategic developments such as DHL's €3.5 billion multiyear capex plan and UPS's acquisition of Andlauer Healthcare Group further reinforce global clinical supply infrastructure. These expansions aim to improve cryogenic logistics, temperature-controlled warehouses, and advanced distribution routes.
Get a preview of our Clinical Trial Supplies Market Playbook - your guide to GTM strategy, competitive intelligence, supplier dynamics, and Consumer Behavior Analysis: https://marketgenics.co/playbook/clinical-trial-supplies-market-01651
Future Outlook: Opportunities to 2035
The Clinical Trial Supplies Market is expected to generate USD 5.1 billion in new revenue opportunities by 2035, driven by:
Growth of Cold Chain and Cryogenic Logistics
Increasing use of biologics, gene therapies, and cell therapies continues to push demand for advanced storage and temperature-controlled transportation.
Expansion of Biopharmaceutical Packaging
Sustainable, tamper-evident, and trial-ready packaging solutions will grow as regulatory standards tighten.
Digital and Automated Supply Management
IoT sensors, blockchain traceability, and AI forecasting will become mainstream across the Clinical Trial Materials Market.
Rise of Decentralized Trial Models
Direct-to-patient logistics and on-demand delivery models will create new revenue streams.
Real-Time Data Analytics
Improved visibility and automation will support faster, more efficient global trials.
Overall, these trends reflect a future where technology, innovation, and patient-centricity drive the expansion of the global Clinical Research Supplies Market.
Contact:
Mr. Debashish Roy
MarketGenics India Pvt. Ltd.
800 N King Street, Suite 304 #4208, Wilmington, DE 19801, United States
USA: +1 (302) 303-2617
Email: sales@marketgenics.co
Website: https://marketgenics.co
About Us
MarketGenics is a global market research and management consulting company empowering decision makers across healthcare, technology, and policy domains. Our mission is to deliver granular market intelligence combined with strategic foresight to accelerate sustainable growth.
We support clients across strategy development, product innovation, healthcare infrastructure, and digital transformation.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Supplies Market Set to Double by 2035 Amid Rising Global R&D Investments here
News-ID: 4287313 • Views: …
More Releases from MarketGenics India Pvt. Ltd.
APAC Deepfake Detection Market Accelerates as Governments Tighten Digital Trust …
A Market Transforming How the World Verifies Reality
The global deepfake detection technology market, valued at USD 0.6 billion in 2025, is positioned to accelerate at a powerful 37.2% CAGR, reaching USD 15.1 billion by 2035.
This growth is driven by one undeniable truth:
Synthetic media is reshaping the threat landscape faster than humans can recognize it.
Deepfake detection technologies now determine:
How newsrooms verify breaking content
How financial institutions prevent identity-spoofing
How governments protect election integrity
How…
Travel & Expense Management Software Market Signals a Digital Pivot | AI, Cloud …
The Travel and Expense Management (TEM) Market Crossroads | A Sector Accelerating, Repricing Efficiency, and Redrawing the Corporate Spend Map
(Is TEM a Back-Office Tool-or the Operating System of the Next Enterprise Economy?)
For years, the travel and expense management software market lived in the administrative shadows-handed off to finance teams, constrained by spreadsheets, and dismissed as a routine cost-control tool. But the numbers now tell a radically different story.
In 2025, the…
Oilfield Equipment Market hits USD 116.2B in 2025 and grows to USD 156.5B by 203 …
Oilfield Equipment Market | The $156.5B Hardware Backbone of the Global Energy System
Every headline loves clean energy. Yet the global energy mix still demands a brutal truth: oil and gas remain the world's primary supply of heat, mobility, and petrochemicals - and the machines that drill, lift, complete, and produce hydrocarbons continue to define industrial capability.
That's why the Oilfield Equipment Market remains a strategic industry - not a relic.
In 2025,…

Machine Tools Market 2025-2035 | USD 109.9B Growth, CNC & Automation Trends
Machine Tools Market | The $109.9B Intelligence Engine of Global Manufacturing
Factories don't work without machine tools. They shape, cut, drill, grind, and define the physical world around us. Yet most end-products - cars, aircraft parts, electronics housings, surgical devices - never reveal the precision machinery behind them.
The Machine Tools Market is the invisible infrastructure that turns digital models into physical reality.
In 2025, the global Machine Tools Market stands at USD…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…