Press release
Virtual Clinical Trials Market Challenges in Data Privacy Security and Regulatory Compliance
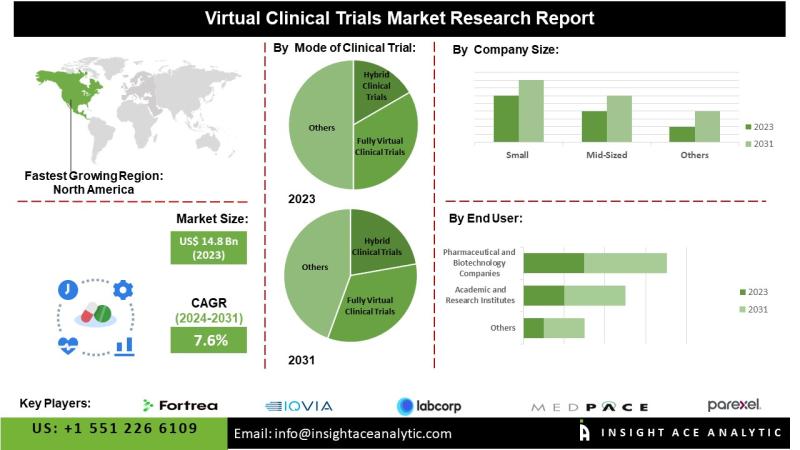
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Virtual Clinical Trials Market Size, Share & Trends Analysis Report Mode Of Clinical Trial (Hybrid Clinical Trial And Fully Virtual Clinical Trial), Study Type (Interventional, Observational), Type Of Therapeutic Area (Cardiovascular Disorders, Infectious Diseases, Metabolic Disorders, Neurological Disorders, Oncological Disorders, Respiratory Disorders), Clinical Trial Phase (Phase I, Phase II, Phase III And Phase IV), Company Size (Small, Mid-Sized And Large), End-User (Pharmaceutical And Biotechnology Companies, Academic And Research Institutes, Medical Device Industries), Region, Market Outlook And Industry Analysis 2031"The Global Virtual Clinical Trials Market is estimated to reach over USD 26.3 billion by 2031, exhibiting a CAGR of 7.6% during the forecast period.
Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/2509
The virtual clinical trials market is witnessing robust growth, driven by advancements in digital health technologies and the rising prevalence of chronic diseases, which have amplified the need for more efficient and cost-effective clinical research models. By integrating remote monitoring, telemedicine, and digital data collection tools, virtual clinical trials enhance accessibility and convenience for both participants and investigators, while addressing traditional challenges such as geographic limitations, recruitment inefficiencies, and participant retention issues.
Nevertheless, the market faces certain challenges, including data privacy and security concerns, complex regulatory compliance requirements, and disparities in access to digital infrastructure. The COVID-19 pandemic acted as a catalyst for the adoption of virtual trial methodologies, demonstrating their capacity to maintain research continuity during global disruptions. In response, industry stakeholders are increasingly investing in sophisticated digital platforms and pursuing strategic partnerships to strengthen technological capabilities and expand market reach.
Looking forward, the virtual clinical trials market is expected to sustain strong growth, supported by continuous technological innovation and the broader shift within the clinical research sector toward patient-centric, decentralized trial models.
List of Prominent Players in the Virtual Clinical Trials Market:
• ICON, plc
• Parexel International Corporation
• IQVIA
• Covance
• PRA Health Sciences
• LEO Innovation Lab
• Medidata
• Oracle
• CRF Health
• Clinical Ink
• Medable, Inc.
• Signing Health
• Halo Health Systems
• Croprime
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2025-02
Market Dynamics: Virtual Clinical Trials
Drivers
The increasing prevalence of chronic diseases is a key factor driving the growth of the virtual clinical trials market. This trend has accelerated the adoption of research methodologies that prioritize efficiency, cost-effectiveness, and patient-centric approaches. Virtual clinical trials leverage remote technologies to enhance accessibility, streamline data collection, and expedite the drug development process, ultimately supporting improved patient outcomes.
Their growing adoption is further reinforced by operational benefits such as reduced costs, improved process efficiency, and enhanced patient recruitment and retention. Features including remote participation, minimized travel requirements, real-time data monitoring, and improved adherence significantly contribute to market expansion. Collectively, these advantages facilitate greater patient engagement and enable more agile execution of clinical research.
Challenges
Despite promising growth prospects, the market faces several constraints. Data privacy and security remain critical concerns, as protecting sensitive patient information against cyber threats is essential. In addition, regulatory frameworks are complex and vary across regions, creating compliance challenges for stakeholders. Limited access to digital infrastructure, including device availability and reliable internet connectivity, further hinders adoption. Moreover, logistical and financial challenges associated with the distribution and administration of at-home diagnostic tools and medications can restrict scalability and broader market penetration.
Regional Trends
North America is anticipated to retain its leading position in the global virtual clinical trials market, supported by advanced healthcare infrastructure, a strong presence of industry leaders, and favorable regulatory frameworks that encourage digital health innovation. Population growth, urbanization, and industrial development further reinforce the region's market dominance. The Asia-Pacific region, meanwhile, is projected to experience substantial growth, driven by proactive initiatives from key industry participants. Ongoing digital healthcare transformation, increasing collaboration among stakeholders, and rising investments from pharmaceutical and biotechnology companies in virtual trial technologies are expected to enhance the region's market potential significantly.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customisation/2509
Recent Developments:
• In July 2023, Signant Health completed the acquisition of DSG, strategically augmenting its eClinical solution suite for both traditional and decentralized clinical trials. By integrating DSG's unified platform, the acquisition facilitated the development of a comprehensive trial ecosystem equipped with advanced software, analytics, and logistics solutions. This enabled seamless study conduct and data generation across all modalities, thereby accomplishing the goal of fully digitalizing clinical trials.
• In June 2023, Medable Inc. unveiled a comprehensive toolkit tailored for Institutional Review Boards (IRBs)/Ethics Committees (ECs), designed to establish standardized ethics review procedures for decentralized clinical trials (DCTs). The implementation of this toolkit successfully simplified, streamlined, and accelerated the IRB/EC process, playing a pivotal role in fostering enhanced efficiency and patient-centeredness in the execution of DCTs.
• In October 2022, Oracle and ObvioHealth entered into a strategic collaboration to integrate diverse data sets into virtual/decentralized clinical trials in the Asia Pacific region. This initiative is expected to allow the quick collection, integration, and analysis of multi-source data collected from labs, devices, patients, and sites.
Segmentation of Virtual Clinical Trials Market-
By Mode of Clinical Trial
• Hybrid Clinical Trial
• Fully Virtual Clinical Trial
By Study Type
• Interventional
• Observational
• Expanded Access
By Type of Therapeutic Area
• Cardiovascular Disorders
• Infectious Diseases
• Metabolic Disorders
• Neurological Disorders
• Oncological Disorders
• Respiratory Disorders
• Other Disorders
By Clinical Trial Phase
• Phase I
• Phase II
• Phase III
• Phase IV
By Company Size
• Small
• Mid-sized
• Large
By End Users
• Pharmaceutical and Biotechnology Companies
• Academic and Research Institutes
• Medical Device Industries
• Other End Users
By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• Southeast Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
View Overview Report: https://www.insightaceanalytic.com/report/virtual-clinical-trials-market/2509
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: https://www.insightaceanalytic.com/
Tel : +1 607 400-7072
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Virtual Clinical Trials Market Challenges in Data Privacy Security and Regulatory Compliance here
News-ID: 4286896 • Views: …
More Releases from Insightace Analytic Pvt Ltd.
Automotive Lead Acid Battery Market Strategic Growth Drivers and Outlook 2026 to …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Lead Acid Battery Market Size, Share & Trends Analysis Report By Product (SLI and Micro-Hybrid Batteries), Type (Flooded, Enhanced Flooded, and VRLA), Customer Segment (OEM and Aftermarket), End User (Passenger Car, Light Commercial Vehicles, Heavy Commercial Vehicles, Two-Wheeler, and Three-Wheeler), and Application (Hybrid Vehicles, Electric Vehicles, Light Motor Vehicles, and Heavy Motor Vehicles)- Market…
Automotive Interior Market Investment Opportunities and Forecast 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Interior Market- (By Component Type (Center Stack, Head-up Display, Instrument Cluster, Rear Sear Entertainment, Dome Module, Headliner, Seat, Interior Lighting Door Panel, Center Console, Adhesives & Tapes, Upholstery, Others), By Material (Leather, Fabric, Vinyl, Wood, Glass Fiber Composite, Carbon Fiber Composite, Metal), By Level of Autonomy (Semi-Autonomous, Autonomous, Non-Autonomous),By Electric Vehicle (Battery Electric Vehicle…
Artificial General Intelligence Market Future Landscape and Industry Evolution 2 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Artificial General Intelligence (AGI) Market - (By Type of Offering (Hardware, Software and Service), Type of Technology (Machine Learning, Deep Learning, Natural Language Processing and Robotics), Mode of Deployment (Cloud-based, On Premise and Web-based), Type of AI (Weak AI, Strong AI and Superintelligence), Type of Processing (Image, Text and Voice Processing), Company Size (SMEs and…
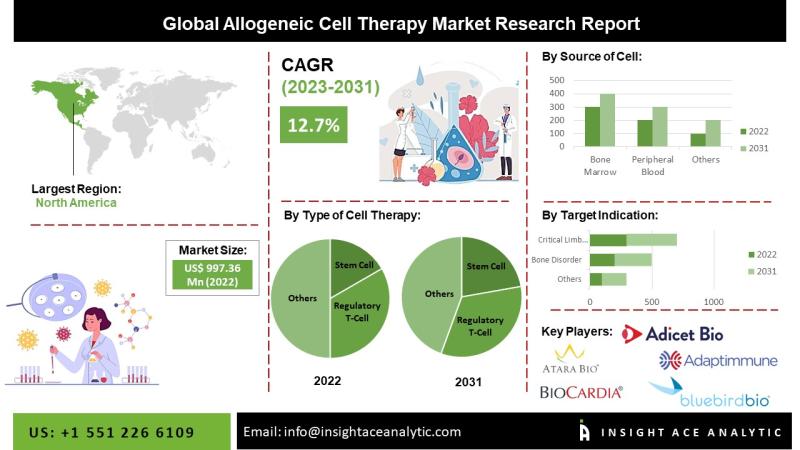
Allogenic Cell Therapies Market Revenue Trends and Growth Potential 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Allogenic Cell Therapies Market- by Cell Type(Cardiosphere-Derived Cells (CDCs), Fibroblasts, T-cells, Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs) and Others),Tissue Source(Skin, Blood, PBC, BM and Others), Indication (Acute graft-versus-host disease (GVHD), Chronic Ulcers and Diabetic Foot Ulcers, Osteoarthritis, Crohn's Disease, Cardiovascular Disease, Solid Tumors/Cancers and Others (Alzheimer's Disease, etc.)), Trends, Industry Competition Analysis, Revenue…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…