Press release
Viral Vector Manufacturing Market Poised for 23.5% CAGR Growth Through 2032 - Persistence Market Research Reports
The viral vector manufacturing market is entering a defining period shaped by surging demand for gene therapies, cell therapies, and next-generation vaccines that rely on safe, high-yield vector systems. As regulatory approvals accelerate and commercial-scale therapies begin reaching broader patient populations, manufacturing capacity has become one of the most critical determinants of market success. With the global market expected to grow from US$1.8 billion in 2025 to US$8.0 billion by 2032, the industry is undergoing rapid transformation driven by technological innovation, bioprocess standardization, and unprecedented investment in contract development and manufacturing organizations (CDMOs).The rapid expansion of viral vector capacity is strongly supported by developments across oncology, rare diseases, and chronic conditions where gene-modifying interventions offer curative potential. AAV vectors account for the largest share of demand, while lentiviral systems-at the heart of CAR-T and engineered immune cell therapies-are expanding at an unmatched pace. Regionally, North America leads the market with 54% share, due to its robust clinical pipeline, strong regulatory environment, and extensive biomanufacturing infrastructure. By contrast, Asia Pacific is the fastest-growing region, propelled by aggressive government investment in China, India, and South Korea to establish advanced therapy ecosystems.
Download Your Free Sample & Explore Key Insights: https://www.persistencemarketresearch.com/samples/26923
These trends are converging to reshape the global viral vector landscape, establishing manufacturing as both a strategic bottleneck and a high-growth opportunity for stakeholders across the pharmaceutical, biotechnology, and CDMO sectors.
Key Highlights from the Report
• The viral vector manufacturing market is projected to expand at a CAGR of 23.5% from 2025-2032.
• AAV vectors accounted for the leading virus type with 45% share in 2025.
• Lentiviral vectors are the fastest-growing vector type, driven by the CAR-T therapy pipeline.
• Gene therapy remains the dominant application with 47% market share in 2025.
• North America leads geographically with 54% global share in 2025, backed by clinical trial concentration.
• CDMOs are the fastest-growing end-user segment due to flexible outsourcing demand.
Market Overview
The viral vector manufacturing market has evolved from a niche bioprocessing segment to a foundational pillar of the global cell and gene therapy (CGT) industry. The sharp increase in approved gene therapies-especially for rare diseases, inherited disorders, and hematologic cancers-has intensified the need for scalable and reproducible vector supply. Viral vectors such as AAV, lentivirus, adenovirus, and retrovirus remain indispensable due to their ability to deliver genetic material with high efficiency and controlled expression profiles.
As of 2025, the market is valued at US$1.8 billion, fueled by advancements in regulatory approval pathways, improvements in vector design, and a steep rise in CGT-focused clinical trials. By 2032, the market is expected to reach US$8.0 billion, driven by commercial-scale manufacturing of gene therapies, expanded reimbursement coverage, and maturation of platform technologies enabling predictable, GMP-compliant vector production.
From a segmentation perspective, AAV leads the virus type category, while gene therapy leads application demand, reflecting a growing number of marketed products targeting neuromuscular, ophthalmic, and hematologic disorders. Geographically, North America remains the dominant region due to its vast clinical pipeline, FDA facilitation programs, and investments from major manufacturers. Meanwhile, Asia Pacific is emerging as the fastest-growing region, supported by national-level biotech programs, growing scientific capability, and significantly lower production costs.
Market Segmentation
Virus Type Segmentation
Virus type segmentation is central to understanding the dynamics of the viral vector manufacturing landscape because each vector class serves distinct therapeutic modalities. AAV vectors dominate the field due to their favorable safety profile, minimal immunogenicity, and ability to support long-term gene expression without integrating into the host genome. These properties have made AAV the backbone of in vivo therapies, particularly for spinal muscular atrophy, hemophilia, retinal disorders, and neurological diseases. Manufacturing processes for AAV have expanded significantly, with high-capacity bioreactors and improved purification technologies enabling clinical and commercial scale production.
Lentiviral vectors, on the other hand, remain integral to ex vivo cell therapy platforms, especially CAR-T and NK cell therapies where stable gene integration is critical. This segment is the fastest-growing in the viral vector market owing to rising approvals and next-generation allogeneic cell therapy programs. The development of advanced lentiviral systems-including self-inactivating constructs and high-yield transient transfection processes-has helped overcome earlier biosafety and scalability concerns.
Other viral systems-such as adenoviral vectors, retroviruses, and poxviruses-continue to expand in vaccine development and cancer immunotherapy applications. These vector types provide rapid expression and strong immunogenicity, making them suitable for prophylactic and therapeutic vaccines.
Get Custom Insights Designed for Your Business: https://www.persistencemarketresearch.com/request-customization/26923
Application Segmentation
Gene therapy forms the core application of the viral vector manufacturing market, representing 47% share in 2025. The growing number of approved therapies and expanding late-stage clinical pipeline highlight the transformative power of gene modification technologies for inherited and chronic diseases. With more than a thousand gene therapy clinical trials underway globally, demand for GMP-grade viral vectors has increased exponentially, prompting large-scale facility expansion and CDMO partnerships.
Vaccine development represents the fastest-growing application segment. The validation of viral vector platforms during the COVID-19 pandemic demonstrated the safety, scalability, and adaptability of adenoviral and viral-vectored vaccines in real-world settings. This infrastructure is now being repurposed to accelerate vaccine development for influenza, RSV, malaria, and emerging infectious diseases. Interest in cancer vaccines utilizing viral vector platforms is also gaining momentum, particularly in personalized oncology programs.
In cell therapy, lentiviral and retroviral vector demand continues to rise due to intensifying CAR-T and CAR-NK research pipelines. The broadening application of vector-modified cells for autoimmune diseases, solid tumors, and allogeneic treatment models further accelerates manufacturing needs.
End-User Segmentation
Biotechnology and pharmaceutical companies remain the leading end-users of viral vector manufacturing services, accounting for 52% market share. These organizations invest heavily in proprietary vector technologies, in-house GMP facilities, and vertically integrated CGT development pipelines. However, rising pipeline complexity and manufacturing bottlenecks have driven companies to adopt dual-sourcing models-splitting production between internal facilities and CDMOs.
CDMOs are the fastest-growing end-user segment through 2032, as outsourcing becomes a preferred strategy for small and mid-sized biotech startups lacking the capital to build fully compliant GMP facilities. The growing complexity of global regulatory requirements and the need for rapid scalability have further strengthened CDMO demand. End-to-end service providers offering process development, analytical validation, regulatory support, and clinical-to-commercial scale manufacturing are becoming essential partners for therapy developers accelerating time-to-market.
Regional Insights
North America
North America remains the dominant market for viral vector manufacturing with 54% global share as of 2025. The region benefits from a highly advanced scientific ecosystem, robust funding networks, and a high density of CGT clinical trials. The U.S. stands out for its progressive regulatory pathways, including RMAT designation, orphan drug incentives, and frequent FDA interaction meetings that streamline development timelines. Investments from public and private sectors continue to expand capacity, including emerging initiatives in decentralized biomanufacturing and advanced therapy hubs. Canada also contributes significantly, supporting vaccine vector manufacturing and rare disease therapy programs with favorable innovation funding initiatives.
Europe
Europe represents approximately 27% market share and remains a global leader due to its harmonized EMA regulatory framework, world-class scientific talent, and advanced biomanufacturing capabilities. Germany, the U.K., Switzerland, and France are major centers of viral vector production, hosting both CDMOs and global pharma manufacturers. While the region benefits from regulatory alignment across 27 EU member states, pricing and reimbursement challenges continue to limit broad commercial adoption of some high-cost gene therapies. Despite this, Europe remains a critical hub for lentiviral and AAV vector development, supported by strong industrial-academic collaborations and specialized expertise.
Asia Pacific
Asia Pacific is the fastest-growing viral vector manufacturing region, forecast to expand at a 22% CAGR through 2032. China leads with massive government-backed CGT investments under national biotech strategies. Streamlined regulatory pathways and cost-effective manufacturing continue to attract global partnerships. India is emerging as a key manufacturing destination with growing biologics capability, tariff advantages, and government incentives for advanced therapy facilities. Japan, South Korea, and Singapore are also accelerating investments to strengthen their positions in the global CGT market.
Market Drivers
The primary driver of the viral vector manufacturing market is the rapid acceleration of gene therapy approvals for rare and inherited disorders. Regulatory agencies such as the FDA and EMA have introduced expedited approval pathways, significantly reducing development timelines and enhancing the commercial feasibility of high-value therapies. Evolving reimbursement models-including value-based payment mechanisms and outcomes-linked compensation-are supporting the widespread adoption of one-time curative treatment models. Furthermore, increased investments from pharmaceutical companies, growing venture capital support for CGT startups, and heightened global awareness of genetic disease interventions continue to expand the demand for high-volume GMP-grade vector manufacturing.
Market Restraints
Despite robust growth, the viral vector manufacturing market faces key challenges. The biological complexity of vector production creates inherent inconsistencies that complicate batch reproducibility and quality control. High capital expenditure for GMP facilities, specialized workforce needs, and stringent regulatory requirements elevate manufacturing costs and create barriers for small biotech firms. Differences in FDA and EMA potency testing standards further complicate global commercialization strategies, requiring dual analytical testing and increasing development timelines. Additionally, raw material shortages, plasmid supply limitations, and purification bottlenecks continue to hinder industry scalability.
Market Opportunities
AI-enabled bioprocess optimization is emerging as one of the most transformative opportunities in viral vector manufacturing. Machine learning models are being integrated into real-time bioreactor monitoring systems to enhance yield prediction, optimize transfection efficiency, and minimize production variability. Downstream processes are also benefiting from computational modeling, reducing experimental burden and accelerating commercialization timelines. Platform technologies, including standardized cell lines, media, and transfection kits, are enabling modular, reproducible manufacturing systems ideal for multi-program scaling. CDMOs adopting these standardized platforms can service diverse clients efficiently, lowering costs and accelerating therapy availability across global markets.
Checkout Now & Download Complete Market Report: https://www.persistencemarketresearch.com/checkout/26923
Company Insights
• Thermo Fisher Scientific Inc.
• Lonza Group AG
• Merck KGaA
• Catalent, Inc.
• Sartorius AG
• Oxford Biomedica plc
• FUJIFILM Diosynth Biotechnologies
• Charles River Laboratories International, Inc.
• WuXi Biologics (Cayman) Inc.
• AGC Biologics
• Novartis AG
• Takara Bio Inc.
• Aldevron, LLC
• Batavia Biosciences B.V.
• Resilience
Market Segmentation
By Virus Type
Adeno-Associated Virus (AAV) Vectors
Lentiviral Vectors
Adenoviral Vectors
Retroviral Vectors
Others
By Application
Gene Therapy
Vaccine Development
Cancer Therapy
Research & Development
Others
By End-user
Biotechnology Companies
Pharmaceutical Companies
Contract Development & Manufacturing Organizations (CDMOs)
Academic & Research Institutes
By Region
North America
Europe
East Asia
South Asia & Oceania
Latin America
Middle East & Africa
Recent Industry Developments
October 2025: Australia launched its first large-scale viral vector manufacturing facility in Sydney, featuring GMP-grade AAV and lentiviral production capacity up to 500L, strengthening the region's CGT manufacturing ecosystem.
September 2025: DINAMIQS opened a state-of-the-art cGMP facility in Zurich with 1,000L single-use manufacturing capacity, enhancing Europe's vector scalability and reducing clinical-to-commercial transition timelines.
Conclusion
The viral vector manufacturing market is undergoing profound expansion as gene therapies and advanced vaccines transition from emerging innovations to mainstream therapeutic solutions. With the global market projected to reach US$8.0 billion by 2032, manufacturing scalability, regulatory harmonization, and technological innovation will determine competitive advantage. Regions such as North America and Europe will continue to drive scientific advances, while Asia Pacific will grow rapidly as a cost-effective production hub. As AI-enabled bioprocessing, platform standardization, and CDMO expansion continue reshaping manufacturing economics, the viral vector industry is set to become a foundational pillar of next-generation medicine, enabling broader patient access and accelerating breakthroughs in genetic and cellular therapeutics.
Read More Related Reports:
Extracellular Matrix Patches Market https://www.persistencemarketresearch.com/market-research/extracellular-matrix-patches-market.asp
Human Immunodeficiency Virus Therapeutics Market https://www.persistencemarketresearch.com/market-research/human-immunodeficiency-virus-therapeutics-market.asp
Cell Line Development Market https://www.persistencemarketresearch.com/market-research/cell-line-development-market.asp
Idiopathic Thrombocytopenic Purpura Therapeutics Market https://www.persistencemarketresearch.com/market-research/idiopathic-thrombocytopenic-purpura-therapeutics-market.asp
Contact Us:
Persistence Market Research
Second Floor, 150 Fleet Street, London, EC4A 2DQ, United Kingdom
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Viral Vector Manufacturing Market Poised for 23.5% CAGR Growth Through 2032 - Persistence Market Research Reports here
News-ID: 4286068 • Views: …
More Releases from Persistence Market Research
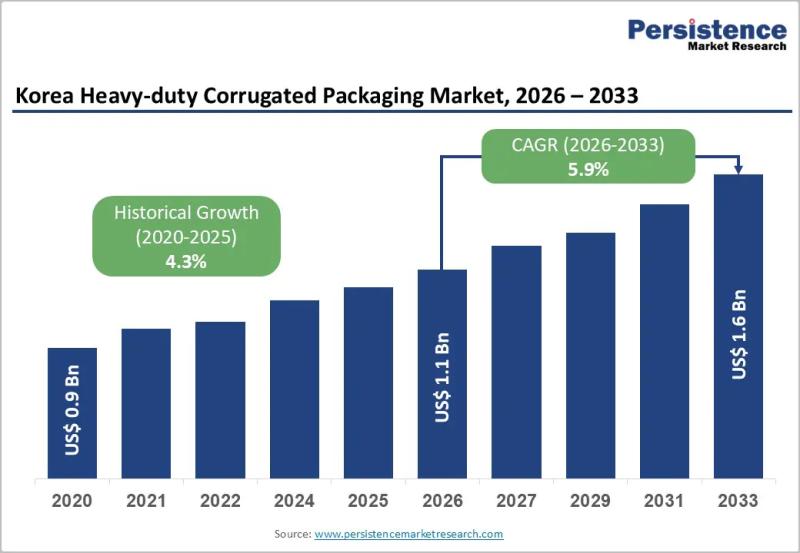
Korea Heavy-duty Corrugated Packaging Market to Reach US$1.6 Billion by 2033 - P …
The Korea heavy-duty corrugated packaging market plays a critical role in supporting industrial logistics, bulk transportation, and export-driven manufacturing. Heavy-duty corrugated packaging is widely used for shipping machinery, automotive components, electronics, chemicals, and large industrial goods that require superior strength and structural integrity. Unlike conventional corrugated boxes, heavy-duty variants are engineered with multi-wall boards, reinforced liners, and customized structural designs to withstand high load capacity, stacking pressure, and long-distance transportation.…

Textile Flooring Market Set for Steady Growth as Demand for Sustainable and Styl …
The global textile flooring market is entering a phase of stable expansion, supported by rising construction activity, increasing consumer focus on interior aesthetics, and growing demand for eco-friendly flooring solutions. According to industry estimates, the global textile flooring market size is likely to be valued at US$11.1 billion in 2026 and is projected to reach US$16.5 billion by 2033, expanding at a CAGR of 5.8% between 2026 and 2033. This…

Power System Simulator Market Size to Reach US$ 2.6 Billion by 2033 - Persistenc …
The power system simulator market is gaining strategic importance as global energy systems transition toward digitalization, decentralization, and decarbonization. Power system simulators are advanced software and hardware platforms used by utilities, grid operators, engineering firms, and research institutions to model, analyze, and optimize electrical power networks. These simulators enable real time grid analysis, contingency planning, load flow studies, fault analysis, stability assessment, and operator training. As electricity networks become more…

Yoga and Meditation Products Market Set for Robust Growth, Projected to Reach US …
The global wellness industry is undergoing a major transformation as consumers increasingly prioritize mental health, mindfulness, and preventive self-care. Within this evolving landscape, the yoga and meditation products market has emerged as a fast-growing segment, encompassing everything from yoga mats and apparel to meditation cushions, smart devices, and digital-enabled accessories. According to industry estimates, the global yoga meditation products market is projected to be valued at US$ 8.3 billion in…
More Releases for CDMO
FDP CDMO Research: China FDP CDMO market size is projected to reach USD 1.33 bil …
QY Research Inc. (Global Market Report Research Publisher) announces the release of 2025 latest report "Fraud Detection and Prevention (FDP) System- Global Market Share and Ranking, Overall Sales and Demand Forecast 2025-2031". Based on current situation and impact historical analysis (2020-2024) and forecast calculations (2025-2031), this report provides a comprehensive analysis of the global Wire Drawing Dies market, including market size, share, demand, industry development status, and forecasts for the…
Global Cmo And Cdmo Biotechnology Market Size by Application, Type, and Geograph …
According to Market Research Intellect, the global Cmo And Cdmo Biotechnology market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
Biologics and sophisticated medicines are driving the biotechnology industry for Contract Manufacturing Organizations (CMO) and Contract…
Evolving Market Trends In The Inhalation CDMO Industry: Strategic Collaborations …
The Inhalation CDMO Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Expected Inhalation CDMO Market Size During the Forecast Period?
In recent times, the inhalation CDMO market has experienced significant growth. The market value is expected to increase from $2.08 billion in 2024…
What's Driving the Inhalation CDMO Market 2025-2034: Rising Respiratory Disorder …
How Is the Chondroplasty Market Projected to Grow, and What Is Its Market Size?
The chondroplasty market has seen strong growth in recent years. It will increase from $13.77 billion in 2024 to $14.68 billion in 2025 at a CAGR of 6.5%. This growth is attributed to the rise in sports-related injuries, patient preference for non-total joint replacement procedures, advances in postoperative care, healthcare provider training, and an increasing incidence of…
Lentiviral Vector (LVV) CDMO Services Market Delivering Cures: The Role of LVV C …
Lentiviral Vector (LVV) CDMO Services Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Lentiviral Vector (LVV) CDMO Services Market - (By Type (IIT Grade, IND Grade, Clinical Trial Grade, Commercial Production Grade), By Application (Biopharmaceutical Company, Academic Scientific Research Institution)), Trends, Industry Competition Analysis, Revenue and Forecast To…
Electronic Chemicals CDMO Market Fueling the Electronics Boom: The Rise of the E …
Electronic Chemicals CDMO Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Electronic Chemicals CDMO Market - (By Type (Metals and Pastes, Electronic Specialty Gases, Polymer Compounds, Others), By Application (Battery, Semiconductor, Integrated Circuit, Consumer Electronics, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest…
