Press release
Clinical Trial Services Market Surges at 7.3% CAGR; North America Dominates with 43% Share
The Clinical Trial Services Market continues to demonstrate strong and sustained momentum as the life sciences industry undergoes rapid digital transformation, rising therapeutic complexity, and a global push for faster, more cost-efficient drug development. Valued at USD 62.2 billion in 2025, the market is projected to reach approximately USD 135 billion by 2035, expanding at a CAGR of 7.3% during the forecast period. With North America holding 43% market share, the sector is evolving into one of the most strategically important pillars of the global healthcare and biopharmaceutical ecosystem.As clinical protocols grow more technologically intensive, diverse, and globally distributed, sponsors are increasingly outsourcing critical research functions to specialized partners who can deliver scale, precision, and data-driven decision support. This shift is reshaping the Clinical Trial Services Market, reinforcing the role of contract research organizations (CROs) and integrated service providers in modern drug development.
In recognition of Black Friday, we are offering a special 15% discount on this comprehensive research report, providing stakeholders with exceptional value and strategic intelligence.
Buy Now: https://marketgenics.co/buy/clinical-trial-services-market-42377
Market Overview: Structure, Evolution, and Strategic Shifts
The Clinical Trial Services Market is moderately consolidated, led by global Tier-1 CROs such as IQVIA, Labcorp Drug Development, PPD, Parexel, and ICON, which together account for over 35% of total market activity. These organizations provide end-to-end capabilities spanning protocol development, site management, patient engagement, logistics, monitoring, clinical data management, and analytics.
The market structure continues to evolve in three major ways:
Digital-first trial execution
Platforms integrating eCOA, eConsent, wearables, telehealth, and decentralized clinical trial (DCT) models are becoming standard components of trial delivery.
AI-enabled workflows
CROs are investing heavily in artificial intelligence to accelerate patient matching, risk-based monitoring, imaging analysis, and literature review.
Real-world evidence (RWE) integration
Disease registries, EHR linkages, and longitudinal databases are supplementing traditional clinical endpoints and enhancing study feasibility.
These shifts make the market increasingly intertwined with advanced analytics, high-performance computing, and digital pathology-capabilities that are reshaping competitive differentiation across the Clinical Research Services Market.
Get the Detailed Industry Analysis (including the Table of Contents, List of Figures, and List of Tables) - from the Clinical Trial Services Market Research Report: https://marketgenics.co/reports/clinical-trial-services-market-42377
Demand Landscape: Key Growth Drivers
Patient-Centric, Digitally Enabled Trial Models
A major driver of growth in the Clinical Trial Services Market is the shift toward patient-centric trial execution. Hybrid and decentralized designs are reducing site burdens, enabling flexible participation, and improving retention. Industry leaders are integrating:
eConsent and remote onboarding
eCOA for real-time patient reporting
Wearable biosensors generating continuous digital biomarkers
Home health visits to reduce travel
Telemedicine-based assessments
In early 2025, Thermo Fisher Scientific expanded its CorEvitas disease registries to support more robust longitudinal RWE streams, enabling trial sponsors to improve feasibility modeling and enrollment planning. This trend is accelerating across the broader clinical trials market, reflecting a fundamental redesign of how patients engage with research.
Rising Complexity of Phase II and Phase III Protocols
As trial designs incorporate multimodal endpoints-imaging, genomic profiling, digital biomarkers, and AI-supported diagnostics-sponsors require higher levels of operational expertise. This supports sustained demand for:
Risk-based monitoring
Centralized and remote monitoring
Multi-country regulatory navigation
Complex sample logistics
Advanced data management integrations
The growing complexity significantly boosts the relevance of the Clinical Research Services Market, making advanced service portfolios a decisive factor for sponsors.
AI Transformation Across the Trial Lifecycle
In June 2025, IQVIA deployed custom agentic AI systems leveraging NVIDIA platforms to automate target identification, data review, and literature analysis. Similarly, Parexel partnered with Palantir to enhance AI-powered data management and trial logistics. These innovations showcase how AI is compressing development timelines and improving quality assurance across the Clinical Trial Services Market.
To know more about the Clinical Trial Services Market - Download our Sample Report: https://marketgenics.co/download-report-sample/clinical-trial-services-market-42377
Market Restraints: Data Integrity and Interoperability Challenges
Despite rapid progress, the market faces significant challenges related to:
Fragmented EDC, eSource, imaging, and eCOA systems
Calibration drift in imaging and biomarker data
Workflow inconsistencies across decentralized and hybrid models
Backlogs in data reconciliation due to multimodal inputs
CROs are investing in standardized workflows, AI-driven QC tools, and unified trial platforms to address these challenges. However, until the industry achieves full interoperability, the operational complexity of large-scale, multi-vendor studies will continue to constrain efficiency in parts of the clinical trials market.
Opportunity Landscape: RWE, Computational Pathology & External Control Arms
One of the fastest-growing opportunity areas in the Clinical Trial Services Market is the integration of real-world evidence and computational pathology:
Disease-specific registries enable dynamic external control arms
AI-pathology platforms improve patient stratification
Algorithm-driven tissue analytics enhance early signal detection
EHR-integrated enrollment models optimize site selection
In 2025, Labcorp expanded its collaboration with PathAI to operationalize AI-powered pathology algorithms, enabling faster oncology trial development. These innovations are creating new, high-value service lines within the Clinical Research Services Market.
Segmental Analysis: Key Insights Across Major Categories
Service Type
The Clinical Trial Management & Monitoring segment dominates the market with roughly 32% share in 2025. Rapid protocol complexity, decentralized models, and risk-based monitoring requirements are driving demand. ICON, Parexel, and Labcorp have intensified their platform investments to support compliance, transparency, and real-time oversight.
Therapeutic Area
Oncology remains the largest therapeutic segment due to expanding precision medicine pipelines. Other rapidly growing areas include:
Neurology
Immunology
Cardiovascular diseases
Rare diseases
These categories require specialized endpoints and complex datasets, further expanding requirements across the clinical trials market.
Delivery Model
Three primary models are shaping the market:
Full-Service Outsourcing (FSO) for large programs
Functional Service Provider (FSP) arrangements
Hybrid Models combining FSO + FSP
Hybrid models continue gaining momentum, particularly among top pharmaceutical sponsors seeking greater control and modular support.
Geographic Insights
North America leads in demand due to its mature research ecosystem, AI adoption, and strong regulatory infrastructure. Ongoing investments by Labcorp, Pfizer, Thermo Fisher, and Medidata strengthen the region's leadership position in the Clinical Trial Services Market.
Asia Pacific remains the fastest-growing region due to large patient pools, improving site infrastructure, and expanding government support for clinical research.
Get a preview of our Clinical Trial Services Market Playbook - your guide to GTM strategy, competitive intelligence, supplier dynamics, and Consumer Behavior Analysis: https://marketgenics.co/playbook/clinical-trial-services-market-42377
Future Outlook: Market Opportunities Through 2035
The Clinical Trial Services Market is expected to create a cumulative opportunity of USD 72.8 billion from 2025-2035. Key future catalysts include:
Mainstream adoption of decentralized and hybrid trial models
AI-driven protocol design and predictive analytics
Sustainable, site-centric operational frameworks
Expansion of external control arms using high-fidelity RWE
Growth of biologics and medical device trials
Digital pathology integration for real-time decision-making
With trial complexity, global site expansion, and RWE integration accelerating simultaneously, the sector is positioned for enduring relevance within the broader Clinical Research Services Market.
Contact:
Mr. Debashish Roy
MarketGenics India Pvt. Ltd.
800 N King Street, Suite 304 #4208, Wilmington, DE 19801, United States
USA: +1 (302) 303-2617
Email: sales@marketgenics.co
Website: https://marketgenics.co
About Us
MarketGenics is a global market research and management consulting company empowering decision makers across healthcare, technology, and policy domains. Our mission is to deliver granular market intelligence combined with strategic foresight to accelerate sustainable growth.
We support clients across strategy development, product innovation, healthcare infrastructure, and digital transformation.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Services Market Surges at 7.3% CAGR; North America Dominates with 43% Share here
News-ID: 4284855 • Views: …
More Releases from MarketGenics India Pvt. Ltd.
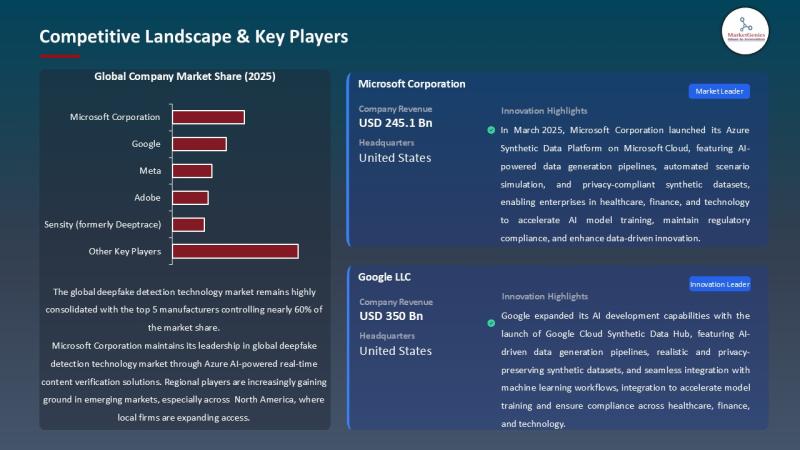
APAC Deepfake Detection Market Accelerates as Governments Tighten Digital Trust …
A Market Transforming How the World Verifies Reality
The global deepfake detection technology market, valued at USD 0.6 billion in 2025, is positioned to accelerate at a powerful 37.2% CAGR, reaching USD 15.1 billion by 2035.
This growth is driven by one undeniable truth:
Synthetic media is reshaping the threat landscape faster than humans can recognize it.
Deepfake detection technologies now determine:
How newsrooms verify breaking content
How financial institutions prevent identity-spoofing
How governments protect election integrity
How…
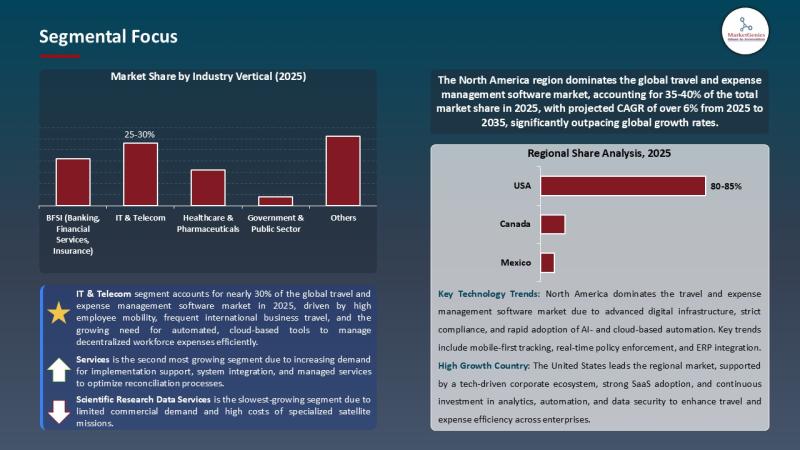
Travel & Expense Management Software Market Signals a Digital Pivot | AI, Cloud …
The Travel and Expense Management (TEM) Market Crossroads | A Sector Accelerating, Repricing Efficiency, and Redrawing the Corporate Spend Map
(Is TEM a Back-Office Tool-or the Operating System of the Next Enterprise Economy?)
For years, the travel and expense management software market lived in the administrative shadows-handed off to finance teams, constrained by spreadsheets, and dismissed as a routine cost-control tool. But the numbers now tell a radically different story.
In 2025, the…
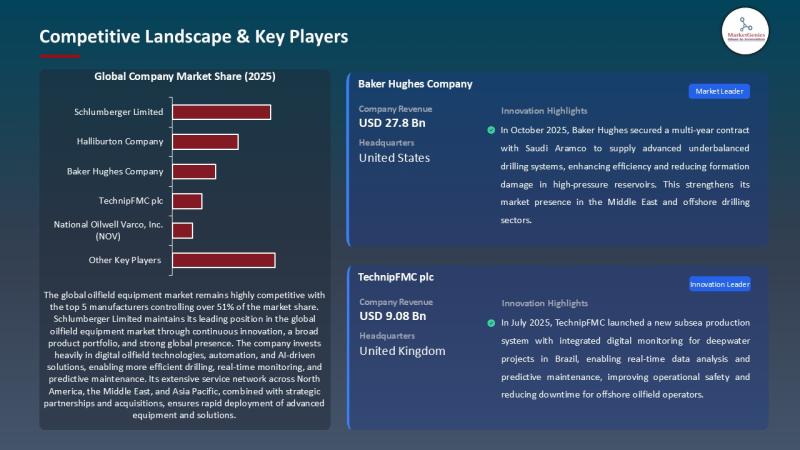
Oilfield Equipment Market hits USD 116.2B in 2025 and grows to USD 156.5B by 203 …
Oilfield Equipment Market | The $156.5B Hardware Backbone of the Global Energy System
Every headline loves clean energy. Yet the global energy mix still demands a brutal truth: oil and gas remain the world's primary supply of heat, mobility, and petrochemicals - and the machines that drill, lift, complete, and produce hydrocarbons continue to define industrial capability.
That's why the Oilfield Equipment Market remains a strategic industry - not a relic.
In 2025,…

Machine Tools Market 2025-2035 | USD 109.9B Growth, CNC & Automation Trends
Machine Tools Market | The $109.9B Intelligence Engine of Global Manufacturing
Factories don't work without machine tools. They shape, cut, drill, grind, and define the physical world around us. Yet most end-products - cars, aircraft parts, electronics housings, surgical devices - never reveal the precision machinery behind them.
The Machine Tools Market is the invisible infrastructure that turns digital models into physical reality.
In 2025, the global Machine Tools Market stands at USD…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…
