Press release
Clinical Trial Software Market Landscape to 2034: Key Forces Shaping the Next Decade of Growth
Use code ONLINE20 to get 20% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.How Large Will the Clinical Trial Software Market Size By 2025?
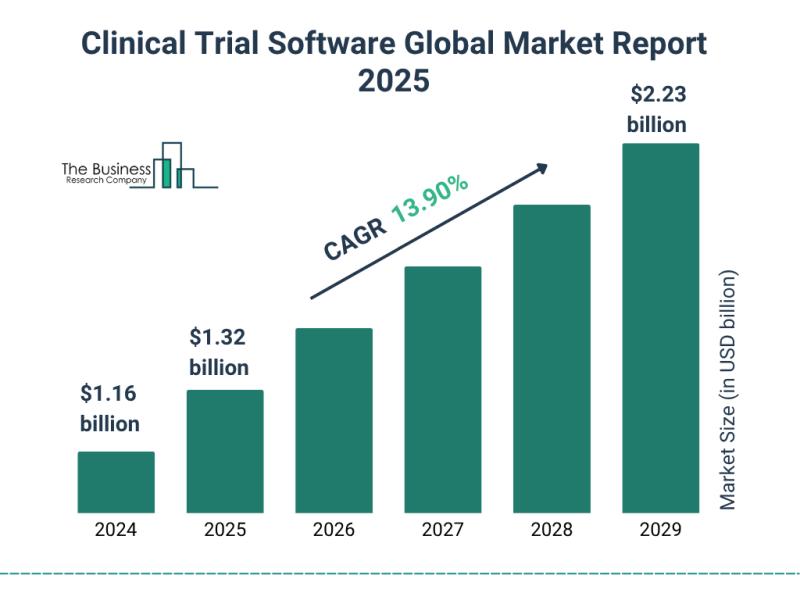
The dimensions of the clinical trial software market have experienced swift expansion lately, projected to escalate from 1.16$ billion in the year 2024 to 1.32$ billion by 2025, reflecting a compound annual growth rate (CAGR) of $14.1%. This expansion witnessed during the past period stems from several factors: a rising requirement for novel software solutions, heightened implementation of contemporary technologies, greater digitalization across clinical trial processes, expanded utilization of cloud-based computing, and a growing population of smaller and medium-sized biopharmaceutical enterprises.
How Big Is the Clinical Trial Software Market Size Expected to Grow by 2029?
Anticipated to experience brisk expansion over the ensuing years, the market encompassing clinical trial software is projected to achieve a valuation of $2.23 billion by 2029, sustaining a compound annual growth rate (CAGR) of 13.9%. This upward trajectory throughout the projection timeframe is fueled by several key factors, including an uptick in clinical research endeavors, the increasing acceptance of virtual trial methodologies, a heightened need for solutions focused on patient needs within clinical trials, a growing requirement for specialized pharmaceuticals, and the greater incidence of venous ailments. Significant developments shaping this market during the forecast period involve advancements in technology, the adoption of artificial intelligence (AI), ongoing innovations and technological progress, the incorporation of machine learning alongside artificial intelligence, and the integration with data derived from real-world settings.
View the full report here:
https://www.thebusinessresearchcompany.com/report/clinical-trial-software-global-market-report
Which Key Market Drivers Powering Clinical Trial Software Market Expansion and Growth?
The expansion of the clinical trial software sector is anticipated to be driven by a surge in research and development endeavors. These systematic processes involve the careful investigation, design, and rigorous testing of novel products or technological solutions aimed at either enhancing current offerings or introducing entirely new ones. A heightened need for innovation across various sectors, brought about by technological leaps, intense market rivalry, and the necessity for ecologically sound approaches, mandates that corporations allocate greater capital toward creating fresh products, methodologies, and services, thereby escalating R&D efforts. Because clinical trials are indispensable for validating novel medications and treatments, the software facilitating these trials lends crucial support to R&D operations by optimizing the organization, deployment, and oversight of such studies. As a specific illustration, data released by the UK's Office for National Statistics indicated that governmental expenditure on research and development within the United Kingdom climbed by 10.5 percent in 2022, reaching £15.5 billion (equivalent to approximately $19.67 billion), compared to the £14.0 billion ($17.77 billion) recorded the prior year, April 2024. Consequently, the growing scope of research and development activities serves as a key impetus for the advancement of the clinical trial software market.
Get your free sample here:
https://www.thebusinessresearchcompany.com/sample.aspx?id=21150&type=smp
Which Fast-Growing Trends Are Poised to Disrupt the Clinical Trial Software Market?
The principal organizations engaged within the clinical trial software arena are concentrating on pioneering advancements, including the creation of unified trial solutions aimed at optimizing data acquisition and synergy, boosting participant involvement, simplifying trial operations, decreasing expenditure, and expediting the duration required for bringing drugs to market. A Unified Trial Solution constitutes an all-encompassing digital architecture engineered to refine and fortify the stages of a clinical study. As a specific illustration, the pharmaceutical giant AstraZeneca plc, headquartered in the UK, brought forth Evinova in November 2023, a system purposed to incorporate artificial intelligence into the realm of clinical investigation. This novel system incorporates distinctive capabilities, prominently featuring automated patient sourcing, which utilizes sophisticated AI calculations to rapidly pair appropriate candidates with the necessary trial prerequisites, thus effecting a substantial shortening of recruitment windows. Moreover, it encompasses forecasting apparatuses that scrutinize both past and current data streams to anticipate patient reactions, subsequently refining both the structure and operational effectiveness of studies. Its intuitive layout encourages superior patient connection through digital venues designated for dialogue and input, thereby facilitating participants' ability to convey their involvement readily.
What Are the Emerging Segments in the Clinical Trial Software Market?
The clinical trial softwaremarket covered in this report is segmented -
1) By Deployment: On-Premises; Web-Based Clinical Trial Software; Cloud-Based Clinical Trial Software; Other Deployments
2) By Software: Electronic Data Capture (EDC); Electronic Clinical Outcome Assessment (eCOA) Or Electronic Patient-Reported Outcome (ePRO); Electronic Informed Consent
3) By End-User: Pharmaceutical And Biotechnology Companies; Contract Research Organizations (CROs); Medical Device Manufacturers; Other End-Users
Subsegments:
1) By On-Premises: Enterprise Clinical Trial Management Systems; Site-Based Clinical Trial Solutions
2) By Web-Based Clinical Trial Software: Electronic Data Capture (EDC) Systems; Clinical Trial Management Systems (CTMS); Randomization And Trial Supply Management (RTSM)
3) By Cloud-Based Clinical Trial Software: Software-as-a-Service (SaaS) Platforms; AI-Powered Clinical Trial Solutions; Remote Monitoring And Decentralized Trial Systems
4) By Other Deployments: Hybrid Clinical Trial Solutions; Custom-Built Clinical Trial Software
Tailor your insights and customize the full report here:
https://www.thebusinessresearchcompany.com/customise?id=21150&type=smp
Who Are the Global Leaders in the Clinical Trial Software Market?
Major companies operating in the clinical trial software market are International Business Machines Corporation, Oracle Corporation, Wipro Limited, Veeva Systems, Medpace Holdings Inc., Medidata Solutions, Signant Health Inc., Clario Inc., Advarra Inc., Celerion Inc., Veristat LLC, Greenphire Inc., Medrio Inc., Arisglobal LLC, Anju Software Inc., ClinCapture Inc., Palleos Healthcare GmbH, OpenClinica LLC, Pharmaseal International Limited, SoftFormance
Which are the Top Profitable Regional Markets for the Clinical Trial Software Industry?
North America was the largest region in the clinical trial software market in 2024. Europe is expected to be the fastest-growing region in the forecast period. The regions covered in the clinical trial software market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Purchase the full report today:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=21150
This Report Supports:
1.Business Leaders & Investors - To identify growth opportunities, assess risks, and guide strategic decisions.
2.Manufacturers & Suppliers - To understand market trends, customer demand, and competitive positioning.
3.Policy Makers & Regulators - To track industry developments and align regulatory frameworks.
4.Consultants & Analysts - To support market entry, expansion strategies, and client advisory work.
Reach out to us:
The Business Research Company: https://www.thebusinessresearchcompany.com/,
Americas +1 310-496-7795,
Europe +44 7882 955267,
Asia & Others +44 7882 955267 & +91 8897263534,
Email us at info@tbrc.info.
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company,
Twitter: https://twitter.com/tbrc_info,
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Learn More About The Business Research Company
With over 17500+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead.Our flagship product, the Global Market Model (GMM), is a premier market intelligence platform delivering comprehensive and updated forecasts to support informed decision-making.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Software Market Landscape to 2034: Key Forces Shaping the Next Decade of Growth here
News-ID: 4280154 • Views: …
More Releases from The Business Research Company
Leading Companies Solidify Their Presence in the Silicone Structural Glazing Mar …
The silicone structural glazing market is positioned for significant expansion in the coming years, driven by advances in building technology and increased environmental awareness. This sector is evolving rapidly as demand grows for more energy-efficient and aesthetically appealing architectural solutions. Let's explore the market's current size, key players, emerging trends, and the main segments that are shaping its future.
Silicone Structural Glazing Market Value Forecast Through 2030
The market for silicone…
Future Prospects: Key Trends Shaping the Self-Healing Concrete Market up to 2030
The self-healing concrete market is capturing significant attention as innovations and sustainability demands rise in construction. This sector is set to experience remarkable growth due to advancements in materials and technology, shaping the future of durable and intelligent infrastructure solutions. Let's explore the market's size, key players, emerging trends, and segment outlook to understand its trajectory.
Projected Market Size and Growth Prospects for the Self-Healing Concrete Market
The self-healing concrete market…
Analysis of Key Market Segments Driving the ROS-Based Robot Industry
The ROS-based robot market is positioned for substantial growth as robotics technology continues to advance rapidly. With increasing innovation in software, hardware, and AI integration, this sector is set to transform multiple industries by 2030. Below, we explore the market's future size, leading companies, key trends, and segmentation details to understand its evolving landscape.
Projected Market Size and Expansion of the ROS-Based Robot Market
The ROS-based robot market is anticipated to…
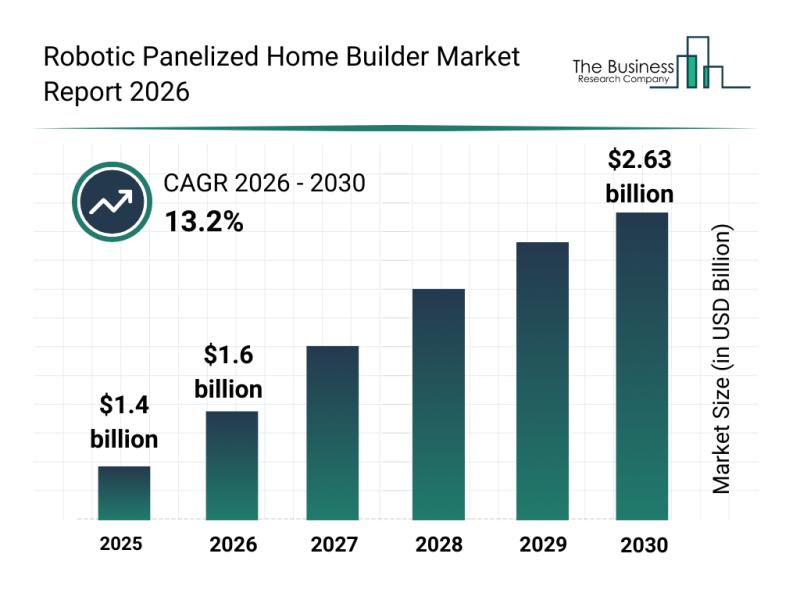
Global Trends Overview: The Rapid Evolution of the Robotic Panelized Home Builde …
The robotic panelized home builder market is positioned for impressive growth in the coming years as automation and robotics increasingly transform construction processes. Driven by technological advancements and expanding prefab housing projects, this market is set to reshape how homes are built with greater speed and efficiency. Let's explore the market's size, leading companies, emerging trends, and key segments that are shaping its future.
Strong Growth Forecast for the Robotic Panelized…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…