Press release
Alport Syndrome Pipeline Outlook Report 2025: MOA, Companies, Companies, Clinical Trials, Expanding Therapeutic Classes, and Future Market Impact Revealed
DelveInsight's "Alport Syndrome Pipeline Insight 2025" report provides comprehensive insights about 4+ companies and 6+ pipeline drugs in the Alport Syndrome pipeline landscape. It covers the Alport Syndrome pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Alport Syndrome pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive Alport Syndrome pipeline products in this space.Explore our latest breakthroughs in Alport Syndrome Research. Learn more about our innovative pipeline today! @ Alport Syndrome Pipeline Outlook [https://www.delveinsight.com/sample-request/alport-syndrome-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Alport Syndrome Pipeline Report
* In September 2025, River 3 Renal Corp. conducted a Phase 2 study to Assess Safety, Tolerability, Efficacy and Pharmacokinetics of R3R01 in Alport Syndrome Patients with Uncontrolled Proteinuria on ACE/ARB Inhibition and in Patients with Primary Steroid-Resistant Focal Segmental Glomerulosclerosis
* DelveInsight's Alport Syndrome pipeline report depicts a robust space with 4+ active players working to develop 6+ pipeline therapies for Alport Syndrome treatment.
* The leading Alport Syndrome Companies such as Enyo Pharma, Bayer, ZyVersa Therapeutics , and others.
* Promising Alport Syndrome Therapies such as Vonafexor, Setanaxib, Bardoxolone Methyl, R3R01, Sparsentan, Atrasentan and others.
Stay informed about the cutting-edge advancements in Alport Syndrome treatments. Download for updates and be a part of the revolution @ Alport Syndrome Clinical Trials Assessment [https://www.delveinsight.com/sample-request/alport-syndrome-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
The Alport Syndrome Pipeline Report provides a disease overview, pipeline scenario and therapeutic assessment of the key pipeline therapies in this domain. The Alport Syndrome Pipeline Report also highlights the unmet needs with respect to Alport Syndrome.
Alport Syndrome Overview
Alport syndrome, a rare genetic disorder affecting around 1 in 50,000 individuals, primarily presents as microscopic hematuria and chronic kidney disease (CKD) with associated extrarenal complications. The Alport syndrome results from mutations in COL4A3, COL4A4, and COL4A5 genes, disrupting the formation of the 3-4-5 chain in the collagen IV network. When the disease first occurs, nonspecific symptoms such as hematuria, proteinuria, and edema appear, making it difficult to differentiate the condition from other kidney diseases, so it might be misdiagnosed.
Alport Syndrome Emerging Drugs Profile
* Vonafexor: Enyo Pharma
Vonafexor (EYP001) is a promising therapeutic candidate developed by Enyo Pharma, specifically designed to treat various kidney diseases, including Alport syndrome and chronic kidney disease (CKD). Vonafexor is a synthetic non-steroidal, non-bile acid farnesoid X receptor (FXR) agonist. It activates FXR with a high selectivity compared to other nuclear receptors and does not display any activity on bile acid receptor TGR5. This small molecule has a different structure compared to other FXR agonists and induces a differential set of target genes based on ligand binding patterns. Currently, the drug is in the Phase II stage of its development for the treatment of Alport Syndrome.
* BAY 3401016: Bayer
BAY 3401016, also known as SEMA 3A, is a monoclonal antibody developed by Bayer in collaboration with Evotec SE. It targets semaphorin 3A (SEMA3A), a protein implicated in various biological processes, including neuronal guidance and immune responses. Currently, the drug is in the Phase I stage of development to treat Alport Syndrome.
The Alport Syndrome Pipeline Report provides insights into
* The report provides detailed insights about companies that are developing therapies for the treatment of Alport Syndrome with aggregate therapies developed by each company for the same.
* It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Alport Syndrome Treatment.
* Alport Syndrome Companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
* Alport Syndrome Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
* Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Alport Syndrome market.
Learn more about Alport Syndrome Drugs opportunities in our groundbreaking Alport Syndrome research and development projects @ Alport Syndrome Unmet Needs [https://www.delveinsight.com/sample-request/alport-syndrome-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Alport Syndrome Companies
Enyo Pharma, Bayer, ZyVersa Therapeutics , and others.
Alport Syndrome Pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
* Oral
* Intravenous
* Subcutaneous
* Parenteral
* Topical
Alport Syndrome Products have been categorized under various Molecule types such as
* Recombinant fusion proteins
* Small molecule
* Monoclonal antibody
* Peptide
* Polymer
* Gene therapy
Discover the latest advancements in Alport Syndrome treatment by visiting our website. Stay informed about how we're transforming the future of Neurology @ Alport Syndrome Market Drivers and Barriers, and Future Perspectives [https://www.delveinsight.com/sample-request/alport-syndrome-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Alport Syndrome Pipeline Report
* Coverage- Global
* Alport Syndrome Companies- Enyo Pharma, Bayer, ZyVersa Therapeutics , and others.
* Alport Syndrome Therapies- Vonafexor, Setanaxib, Bardoxolone Methyl, R3R01, Sparsentan, Atrasentan and others.
* Alport Syndrome Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Alport Syndrome Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
For a detailed overview of our latest research findings and future plans, read the full details of Alport Syndrome Pipeline on our website, @ Alport Syndrome Emerging Drugs and Companies [https://www.delveinsight.com/sample-request/alport-syndrome-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Contents
* Introduction
* Executive Summary
* Alport Syndrome: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Alport Syndrome- DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* Drug Name: Company Name
* Mid Stage Products (Phase II)
* Vonafexor: Enyo Pharma
* Early Stage Products (Phase I)
* BAY 3401016: Bayer
* Preclinical and Discovery Stage Products
* Drug name: Company name
* Inactive Products
* Alport Syndrome Key Companies
* Alport Syndrome Key Products
* Alport Syndrome- Unmet Needs
* Alport Syndrome- Market Drivers and Barriers
* Alport Syndrome- Future Perspectives and Conclusion
* Alport Syndrome Analyst Views
* 22. Alport Syndrome
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=alport-syndrome-pipeline-outlook-report-2025-moa-companies-companies-clinical-trials-expanding-therapeutic-classes-and-future-market-impact-revealed]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/report-store/alport-syndrome-pipeline-insight
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Alport Syndrome Pipeline Outlook Report 2025: MOA, Companies, Companies, Clinical Trials, Expanding Therapeutic Classes, and Future Market Impact Revealed here
News-ID: 4278894 • Views: …
More Releases from ABNewswire

Windy City Chimney Heroes Announces Expanded Service Areas Across the Greater Ch …
Windy City Chimney Heroes, a long-standing chimney company established in 1997, has expanded its service areas across the Greater Chicago region. The company now serves Chicago, Evanston, Oak Park, Naperville, Schaumburg, Cicero, Skokie, Berwyn, and Arlington Heights. Owner Wesley Cook says the expansion will help meet rising demand for reliable chimney repair and inspection services.
Windy City Chimney Heroes, a trusted chimney repair and maintenance provider founded in 1997, has announced…

Injury 2 Wellness Centers Promotes Wellness Care Through Ongoing Chiropractic Su …
As the year draws to a close and many individuals begin setting health goals for the new year, Injury 2 Wellness Centers is encouraging Georgia residents to prioritize wellness care through consistent chiropractic treatment. Far beyond addressing back or neck pain, chiropractic care can serve as a foundation for long-term physical health, improved function, and overall quality of life.
Decatur, GA - December 10, 2025 - As the year draws to…

Avalon Tree Services Reminds Atlanta Homeowners to Prioritize Winter Tree Care f …
As temperatures drop and trees enter their dormant season, Avalon Tree Services is reminding homeowners across Greater Atlanta that winter is one of the most important times to invest in proper tree care. Cold weather, ice accumulation, and seasonal storms can all take a toll on tree health and stability-making preventative maintenance essential for safety and long-term growth.
Atlanta, GA - December 10, 2025 - As temperatures drop and trees enter…
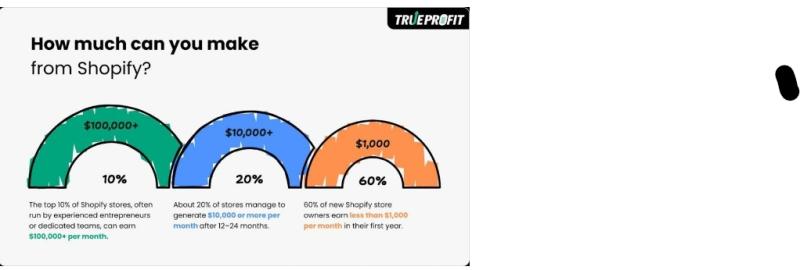
TrueProfit Shares 2026 Guidance for Shopify Startups to Focus on Profit First No …
As Shopify heads into 2026 with millions of active stores worldwide and continued growth in global ecommerce, TrueProfit - a Net Profit Analytics platform for Shopify merchants - is urging new founders to treat profitability as a starting requirement, not an afterthought.
With a low barrier to entry and a mature app ecosystem, Shopify remains one of the easiest ways to launch an online business. But TrueProfit's latest briefing on early-stage…
More Releases for Alport
Alport Syndrome Market Forecasted to Surge in Coming Years, 2024-2034
Market Overview
The Alport Syndrome Market is gradually expanding as rising diagnostic awareness and improved genetic testing enable earlier identification of this rare, inherited kidney disorder. Alport syndrome is primarily caused by mutations in the COL4A3, COL4A4, or COL4A5 genes, affecting type IV collagen and leading to progressive kidney disease, hearing loss, and ocular abnormalities.
Increased adoption of next-generation sequencing (NGS), newborn screening initiatives, and a growing focus on early intervention strategies…
Alport Syndrome Market is Expected to Reach $4.5 Billion by 2034
Alport syndrome is a rare genetic kidney disorder caused by mutations in the COL4A3, COL4A4, or COL4A5 genes, which affect the production of type IV collagen in the basement membranes of the kidneys, inner ear, and eyes. The condition leads to progressive renal disease, hearing loss, and ocular abnormalities, with most patients eventually developing kidney failure requiring dialysis or transplantation.
Download Full PDF Sample Copy of Market Report @ https://exactitudeconsultancy.com/request-sample/71647
Traditionally, treatment…
Alport Syndrome Market Outlook 2034 - Clinical Trials, Market Size, Medication, …
Alport Syndrome companies are Eloxx Pharmaceuticals, River 3 Renal Corp, Chinook Therapeutics, Travere Therapeutics, Reata Pharmaceuticals, and others.
Alport Syndrome Market Summary
The Alport syndrome market in the 7MM was valued at around USD 20 million in 2023 and is projected to grow by 2034. The disorder is primarily X-linked, accounting for 80% of cases, with 90% of males progressing to kidney failure by age 40 if untreated. In 2023, the US…
Alport Syndrome Clinical, Companies, Therapeutic Assessment, Therapies, Treatmen …
Alport Syndrome Pipeline constitutes 4+ key companies continuously working towards developing 6+ Alport Syndrome treatment therapies, analyzes DelveInsight.
Alport Syndrome Overview:
Alport syndrome is a rare inherited disorder, affecting approximately 1 in 50,000 people, and is most often characterized by microscopic hematuria and progressive chronic kidney disease (CKD), along with complications beyond the kidneys. The condition arises from mutations in the COL4A3, COL4A4, and COL4A5 genes, which interfere with the normal assembly…
Alport Syndrome Market Size, Share, Trends, Industry Growth and Competitive Anal …
Data Bridge Market Research analyses a growth rate in the global alport syndrome market in the forecast period 2022-2029.
Market Definition
Alport syndrome is the rare, genetic, inherited X-linked disorder caused by the gene mutation of COL4A3, COL4A4, or COL4A5. It is a condition that damages the tiny blood vessels of the kidney, that leads to kidney damage & failure. It also causes hearing loss and eye abnormalities. Patients suffering from this…
Novel Therapeutic Modalities Transforming the Alport Syndrome Treatment Market 2 …
According to the research report, the global alport syndrome treatment market was valued at USD 12.26 million in 2022 and is expected to reach USD 19.00 million by 2032, to grow at a CAGR of 4.5% during the forecast period.
Polaris Market Research recently launched the latest update on Alport Syndrome Treatment Market: By Size, Trends, Share, Growth, Segments, Industry Analysis and Forecast, 2032 that gives an extensive outlook of the…
