Press release
European Complement 3 Glomerulopathy (C3G) Treatment Market Outlook 2025-2035: Key Developments and Future Scope
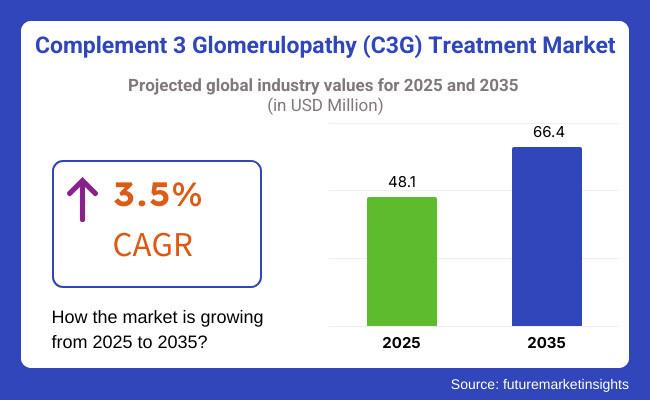
The Complement 3 Glomerulopathy (C3G) Treatment Market is poised for significant growth over the next decade, projected to increase from approximately USD 48.1 million in 2025 to USD 66.4 million by 2035 at a CAGR of 3.5%. This growth is being driven by heightened research and development in orphan kidney disorders, rising diagnostic rates, and the emergence of innovative targeted therapies.Explore trends before investing - request a sample report today!
https://www.futuremarketinsights.com/reports/sample/rep-gb-12461
Advancements in Targeted Therapeutics
Expanding awareness of complement system dysregulation in C3G is fueling market momentum. Novel therapies such as pegcetacoplan and avacopan are increasingly preferred because they address the underlying cause rather than merely mitigating symptoms. Despite the promise of these therapies, limited patient awareness and treatment costs remain barriers, prompting firms to launch early diagnosis and patient assistance programs to enhance access.
Diverse Treatment Modalities Drive Market Expansion
C3G treatment encompasses complement inhibitors, immunosuppressants, plasma therapy, and kidney transplantation. Complement inhibitors, including factor B and C5 inhibitors, are gaining attention for their ability to target the disease at its origin. Factor D inhibitors, like danicopan, are in clinical trials showing promising results, while immunosuppressants such as mycophenolate mofetil (MMF) and corticosteroids deliver variable outcomes. Plasma therapy and transplantation remain secondary options for advanced cases.
Regional Dynamics Shape Growth
North America
The North American market is buoyed by strong healthcare infrastructure and biotech dominance, with the United States leading in FDA-approved drugs and active clinical trials. Key players, including Apellis Pharmaceuticals and Novartis, are heavily investing in targeted therapies. Despite high costs and limited access, reimbursement programs and patient support initiatives are helping bridge the gap.
Europe
Germany leads the European market, followed by France and the United Kingdom, with orphan drug designations facilitating faster approvals. Collaborative efforts between research centers and biotech companies are accelerating innovation, although stringent regulations on biologic pricing continue to challenge adoption. Government-sponsored cost-sharing and access programs are mitigating barriers.
Asia-Pacific
The Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare expenditure, advanced diagnostics, and a rising incidence of rare renal disorders. Japan, China, and India are investing in specialty care and novel complement inhibitors. Early screening campaigns and medical education programs are critical to addressing diagnosis delays and patient unawareness.
Market Challenges and Opportunities
Challenge: Exorbitant Treatment Costs
Complement-targeting biologics, including eculizumab and ravulizumab, present a significant financial barrier. Long-term administration is required, further straining patients and healthcare systems. Price negotiations, reimbursement agreements, and patient assistance programs are being implemented to enhance affordability.
Opportunity: Gene Therapy and Precision Medicine
Breakthroughs in gene editing, such as CRISPR, and precision medicine hold the potential to deliver curative therapies by addressing complement system mutations. Biomarker-based monitoring and highly selective therapeutics are expected to improve efficacy and patient outcomes, creating a paradigm shift in C3G management.
Subscribe for Year-Round Insights → Stay ahead with quarterly and annual data updates -
https://www.futuremarketinsights.com/reports/brochure/rep-gb-12461
Market Trends and Future Outlook
From 2025 to 2035, long-acting complement inhibitors, targeted therapies, and gene-editing treatments will dominate the market. Pharmaceutical companies are focusing on safer, more effective biologics, while regulatory agencies are streamlining approval pathways for orphan kidney disorders. Precision medicine and biomarker-guided treatments are anticipated to redefine patient care globally.
Competitive Landscape
The C3G treatment market is highly competitive, with major players such as Novartis AG, Apellis Pharmaceuticals, ChemoCentryx (Amgen), Alexion Pharmaceuticals (AstraZeneca), and Omeros Corporation leading innovation. Anti-complement therapy remains the most widely adopted treatment due to its targeted mechanism, while cellular immune suppression and plasmatherapy continue as complementary approaches. Hospitals remain the primary end-users, though specialty clinics are increasingly supporting early-stage treatment and patient education.
Buy Report Now - Click Here to Purchase the Report:
https://www.futuremarketinsights.com/checkout/12461
Latest Therapeutic Device Reports
Balloon Catheters for Bile Stone Removal Market
https://www.futuremarketinsights.com/reports/balloon-catheters-for-bile-stone-removal-market
Smart Wheelchair Market
https://www.futuremarketinsights.com/reports/smart-wheelchair-market
Transcatheter Mitral Valve Market
https://www.futuremarketinsights.com/reports/transcatheter-mitral-valve-market
Why Choose FMI: Empowering Decisions that Drive Real-World Outcomes
https://www.futuremarketinsights.com/why-fmi
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware - 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analystsworldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release European Complement 3 Glomerulopathy (C3G) Treatment Market Outlook 2025-2035: Key Developments and Future Scope here
News-ID: 4277574 • Views: …
More Releases from Future Market Insights
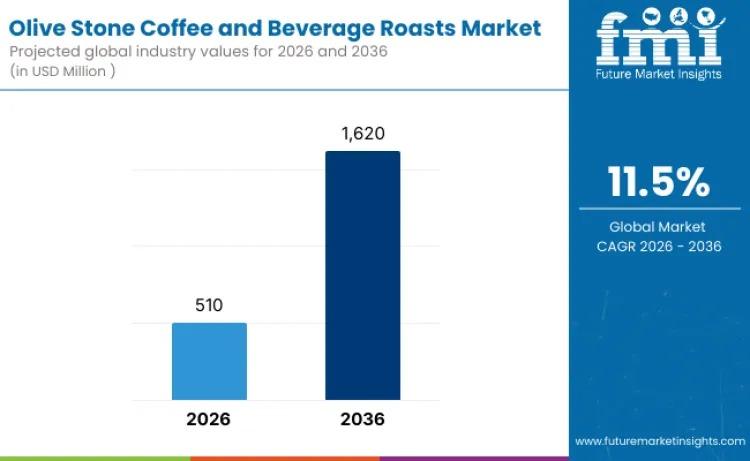
Global Olive Stone Coffee and Beverage Roasts Market to Reach USD 1,620 Million …
The global olive stone coffee and beverage roasts market is entering a high-growth decade, fueled by sustainability innovation and evolving specialty coffee culture. Valued at USD 510 million in 2026, the market is projected to reach USD 1,620 million by 2036, expanding at a compelling CAGR of 11.5%.
As consumers increasingly seek beverages that combine sustainability, functionality, and distinctive taste, olive stone-based roasting solutions are transitioning from niche experimentation to structured…
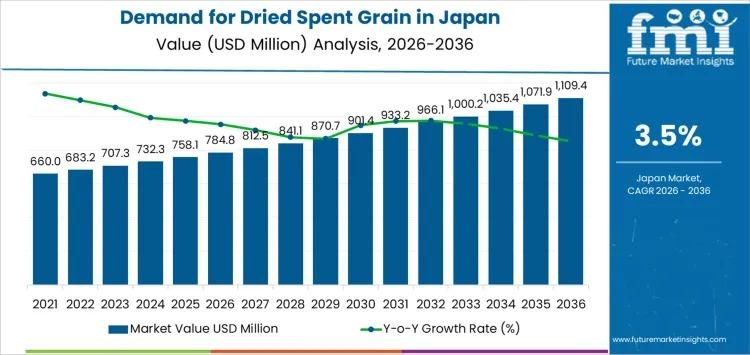
Japan Dried Spent Grain Market to Surpass USD 1.1 Billion by 2036 as Feed Optimi …
Japan's dried spent grain market is entering a decade of steady, value-driven expansion, supported by structured feed demand, brewery byproduct utilization, and rising integration of fiber-rich ingredients into food manufacturing. Industry estimates place the market at USD 784.8 million in 2026, with projections indicating growth to USD 1,109.4 million by 2036, reflecting a CAGR of 3.5%.
Between 2020 and 2026, demand increased from USD 637.5 million to USD 784.8 million, shaped…
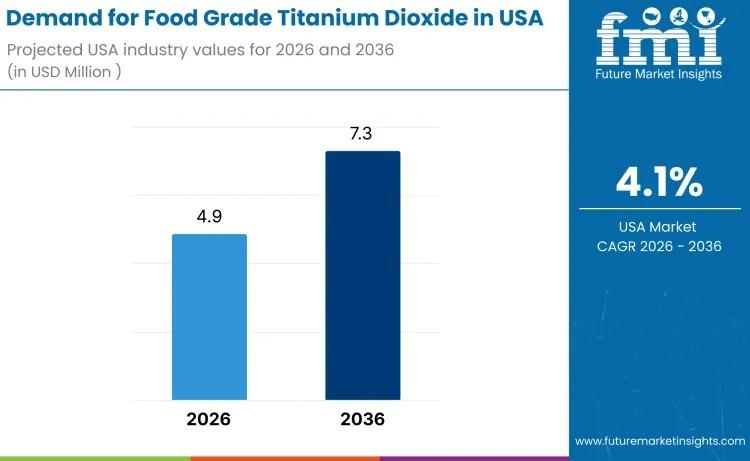
USA Food Grade Titanium Dioxide Market to Reach USD 7.3 Million by 2036 Amid Ste …
The demand for food grade titanium dioxide in the USA is valued at USD 4.9 million in 2026 and is projected to reach USD 7.3 million by 2036, expanding at a CAGR of 4.1%. Growth remains moderate yet stable, supported by continued use of titanium dioxide as a whitening and opacifying agent across confectionery coatings, bakery decorations, sauces, dairy analogues, and processed food matrices.
Despite heightened regulatory scrutiny and evolving clean-label…
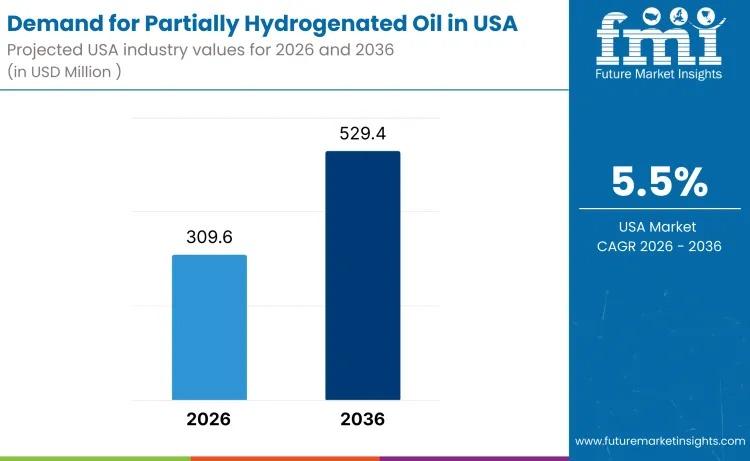
USA Partially Hydrogenated Oil Market to Reach USD 529.4 Million by 2036 Amid Me …
The demand for partially hydrogenated oil in the USA is projected to rise from USD 309.6 million in 2026 to USD 529.4 million by 2036, expanding at a steady CAGR of 5.5%. While edible applications remain tightly regulated, demand persists across specialty industrial and permitted food-related segments where oxidative stability, viscosity control, and texture performance remain critical.
Despite regulatory constraints on trans fats in conventional food manufacturing, PHOs continue to serve…
More Releases for C3G
Complement 3 Glomerulopathy (C3G) Market Expected to Reach USD 2.1 Billion by 20 …
Pune, India, December 2025 - The global Complement 3 Glomerulopathy (C3G) Market is projected to grow from an estimated USD 950 million in 2024 to USD 2.1 billion by 2034, registering a CAGR of 8.4% during the forecast period. The market is driven by advancements in complement inhibition therapies, increased understanding of the disease pathology, and rising diagnosis rates across developed and emerging healthcare systems.
Download Full PDF Sample Copy of…
Pegcetacoplan is approved by the FDA for patients aged 12 and up with rare kidne …
The global Rare Kidney Diseases Market is gaining significant attention as nephrology research, personalized medicine, and orphan drug development continue to expand. Rare kidney diseases such as focal segmental glomerulosclerosis (FSGS), Alport syndrome, Fabry disease, and atypical hemolytic uremic syndrome (aHUS) affect smaller patient populations but often result in high morbidity and limited treatment options. Advances in genetics, biomarker research, and targeted therapies are reshaping the treatment landscape, creating opportunities…
Complement 3 Glomerulopathy (C3G) Market Set to Witness Significant Growth by 20 …
Complement 3 Glomerulopathy (C3G) Market Outlook 2024-2034: Rising Research Focus and Expanding Treatment Pipeline
Introduction
Complement 3 Glomerulopathy (C3G) is a rare kidney disease characterized by abnormal regulation of the complement system, leading to chronic inflammation and progressive kidney damage. Despite its rarity, C3G poses serious challenges to patients, often progressing to end-stage renal disease (ESRD) within a decade of diagnosis.
As awareness of rare renal diseases grows, investment in research and therapy…
Complement 3 Glomerulopathy (C3G) Market Size, Share and Growth Report, 2034
Introduction
The Complement 3 Glomerulopathy (C3G) Market is gaining momentum due to increased recognition of rare kidney diseases, advances in genetic and biomarker testing, and the development of targeted complement pathway inhibitors. C3G, a rare complement-mediated kidney disorder, often leads to progressive renal damage and end-stage kidney disease if untreated. With expanding diagnostic capabilities and the rise of orphan drug development, the market is set for substantial growth over the next…
FDA Approves Fabhalta (Iptacopan) for C3G | Market Impact Across Biopharma, Diag …
FDA Approval of Fabhalta for C3G | Market Impact, Investment Opportunities, and Industry Trends
Published on: 20th March 2025
The U.S. Food and Drug Administration (FDA) has approved Fabhalta (iptacopan), the first-ever treatment for complement 3 glomerulopathy (C3G), a rare kidney disease characterized by excessive proteinuria and progressive kidney damage.
This approval marks a significant milestone in nephrology, with far-reaching implications across various healthcare sectors, including biopharmaceuticals, biotechnology, and diagnostics.
Official Statements on FDA…
Complement 3 Glomerulopathy Market Size was estimated to be USD 91.8 Million in …
The Complement 3 Glomerulopathy Market is set to grow substantially owing to the current treatment practices (mainly off-label treatments), launch of pipeline therapies, increase in funding, better awareness programmes and advent of novel biomarkers for better diagnosis.
The Complement 3 Glomerulopathy market report provides current treatment practices, emerging drugs, Complement 3 Glomerulopathy market share of the individual therapies, current and forecasted Complement 3 Glomerulopathy market Size from 2019 to 2032 segmented…