Press release
PD-1 Inhibitor Drugs Market Outlook to 2035: Investment Opportunities and Growth Path| Akeso Biopharma, Alphamab Oncology, Amgen Inc.
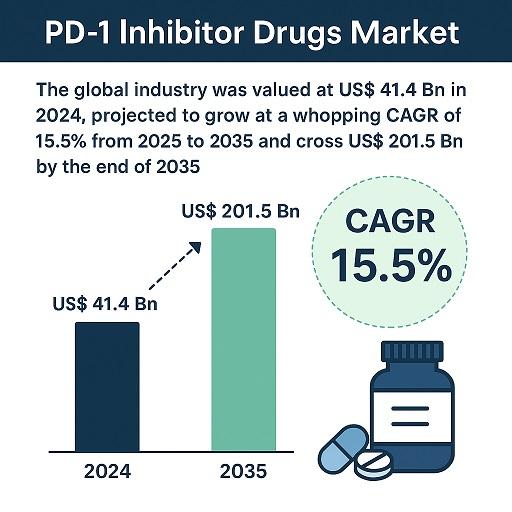
The global PD-1 inhibitor drugs market continues to expand rapidly, driven by strong clinical adoption, innovative combination therapies, and widening oncology applications. The global industry was valued at US$ 41.4 Bn in 2024, supported by increasing demand for effective immunotherapies across major cancer types, including lung cancer, melanoma, and renal cell carcinoma. Looking ahead, the market shows exceptionally strong growth potential as clinical outcomes improve and approvals extend into earlier treatment lines and additional tumor indications. The market is projected to grow at a robust CAGR of 15.5% from 2025 to 2035 and is expected to cross US$ 201.5 Bn by the end of 2035Programmed cell death protein-1 (PD-1) inhibitors have transformed oncology over the past decade by reactivating a patient's immune system to attack cancer cells. These monoclonal antibodies - most famously pembrolizumab and nivolumab - block the PD-1 pathway that tumors exploit to evade immune surveillance. As a class, PD-1 inhibitors now span multiple tumor types and lines of therapy, moving from late-stage, niche indications into earlier lines of treatment and combination regimens.
Get Your Sample Report - Explore Exclusive Insights Now: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=86486
Key Players:
• Akeso Biopharma Co., Ltd.
• Alphamab Oncology
• Amgen Inc.
• AstraZeneca
• BeiGene LTD.
• Bristol-Myers Squibb Company
• Eli Lilly and Company.
• F. Hoffmann-La Roche AG
• Gilead Sciences, Inc.
• GSK plc.
• Innovent
• Jiangsu Hengrui Pharmaceuticals Co., Ltd.
• Merck & Co., Inc.
• Eli Lilly and Company
• Boehringer Ingelheim International GmbH.
• Other prominent players
Key market drivers
Broadened indications and label expansions. Since their initial approvals, PD-1 inhibitors have been approved across a widening range of solid tumors (non-small cell lung cancer, melanoma, renal cell carcinoma, head and neck cancers, certain gastrointestinal cancers) and in some hematologic malignancies. Expansions into adjuvant/neoadjuvant settings and biomarker-driven approvals (e.g., tumor mutation burden, microsatellite instability) continue to increase addressable patient populations.
Combination therapy momentum. Combining PD-1 inhibitors with chemotherapy, targeted therapies, anti-angiogenics, or other checkpoint modulators (e.g., LAG-3 inhibitors) shows additive or synergistic benefit in many tumor types, creating multiple new commercial pathways and prolonging treatment durations.
Biomarker-driven patient selection. Improved diagnostic tests for PD-L1 expression, MSI status, and other immune biomarkers help identify patients most likely to respond, improving clinical outcomes and payor willingness to reimburse.
Strong clinical and commercial backing. Large pharmaceutical companies continue to invest heavily in PD-1 assets and supporting trials, while partnerships and acquisitions fuel pipeline depth.
Market restraints and challenges
High treatment cost and reimbursement pressure. PD-1 therapies are expensive and long-term, creating affordability and access issues worldwide. Health systems and insurers increasingly demand real-world effectiveness and economic value evidence.
Primary and acquired resistance. A meaningful subset of patients do not respond or relapse after initial response. Understanding and overcoming resistance mechanisms remains a major scientific and commercial hurdle.
Safety and management of immune-related adverse events (irAEs). While generally better tolerated than conventional chemotherapy, PD-1 inhibitors can cause potentially severe autoimmune toxicities that require careful monitoring and specialist management.
Competitive saturation and patent cliffs. Leading PD-1 products face biosimilar/biobetter competition over time, and new entrants must differentiate by efficacy, safety, dosing convenience, or cost.
Regional dynamics
North America remains the largest market by revenue due to high per-patient pricing, early adoption, extensive clinical trial activity, and established reimbursement mechanisms.
Europe shows steady uptake but with greater pricing pressure and health technology assessment (HTA) scrutiny that shapes access.
Asia-Pacific is often the fastest-growing region driven by rising oncology incidence, expanding healthcare infrastructure, increasing clinical trial activity, and greater public/private funding for cancer care - though affordability and local regulatory timelines vary by country.
Emerging markets in Latin America, the Middle East, and Africa are at earlier stages of access; growth will depend on price negotiations, local manufacturing or biosimilar introductions, and stronger diagnostic capacity.
Buy this premium research report for valuable insights: https://www.transparencymarketresearch.com/checkout.php?rep_id=86486<ype=S
Trends shaping the future
Precision immunotherapy. Integration of multi-omic biomarkers and AI-driven predictive models is refining patient selection to maximize response rates and reduce unnecessary exposure.
Shift to earlier disease stages. Approvals in adjuvant and neoadjuvant settings increase the pool of patients and potential curative outcomes, but also raise questions about long-term toxicity and health economics.
Oral and engineered alternatives. Research into small molecules, bispecifics, and cell-based therapies aims to either complement or supplant monoclonal antibodies in certain settings.
Real-world evidence (RWE) and value-based contracts. Payers increasingly demand RWE to support outcomes-based reimbursement models, which could influence pricing and formulary placement.
Global access initiatives. Tiered pricing, licensing, and local manufacturing partnerships are being explored to expand access in lower-income countries.
Unlock Comprehensive Market Insights - Download the Full Report Now: https://www.transparencymarketresearch.com/pd-1-inhibitor-drugs-market.html
Opportunities for stakeholders
Pharma and biotech can pursue differentiated indications, novel combinations, and improved delivery formats to capture late adopters and payor support.
Diagnostics firms have a growing role as companion tests become standard; integrated diagnostics-therapy bundles may emerge.
Hospitals and payers can optimize care pathways, toxicity management, and outcome tracking to improve cost-effectiveness.
Investors should watch companies with robust combination pipelines, innovative biomarkers, or manufacturing/price advantages.
Outlook and conclusion
The PD-1 inhibitor market remains one of the most important and dynamic segments in oncology. Continued label expansions, combination strategies, and better biomarker-driven patient selection will sustain growth for the foreseeable future. However, long-term commercial success will depend on demonstrating durable survival benefits, managing costs and safety, and navigating an increasingly crowded therapeutic landscape. For stakeholders who can align clinical innovation with demonstrable value - whether through differentiated products, companion diagnostics, or innovative access models - the PD-1 space offers substantial commercial and patient-impact opportunities as oncology care moves further into precision immunotherapy.
More Trending Reports by Transparency Market Research -
Radiopharmaceuticals Market - https://www.transparencymarketresearch.com/radiopharmaceuticals-market.html
Insulin Market - https://www.transparencymarketresearch.com/insulin-market.html
Microbiology Culture Market - https://www.transparencymarketresearch.com/microbiology-culture-market.html
Wound Healing Market - https://www.transparencymarketresearch.com/wound-healing-market.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release PD-1 Inhibitor Drugs Market Outlook to 2035: Investment Opportunities and Growth Path| Akeso Biopharma, Alphamab Oncology, Amgen Inc. here
News-ID: 4277525 • Views: …
More Releases from Transparency Market Research
Gas Compressor Market Outlook 2036: Global Industry Expected to Reach US$ 41.0 B …
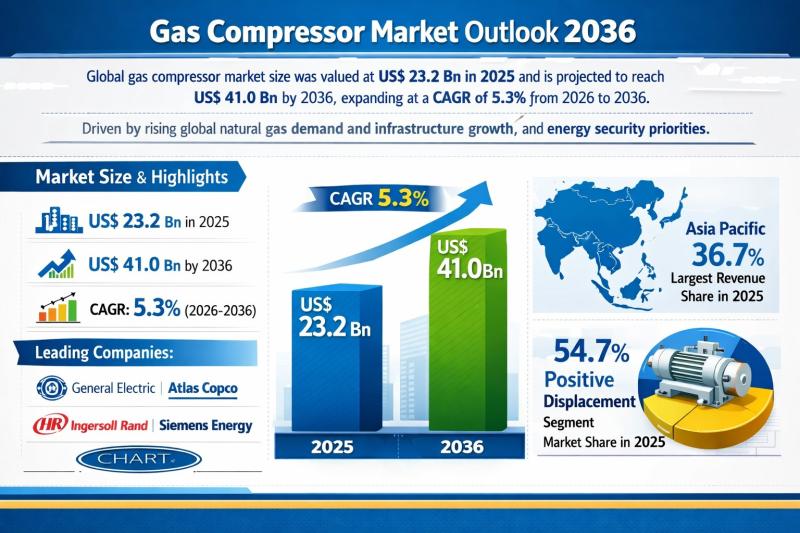
The global gas compressor market was valued at US$ 23.2 Bn in 2025 and is projected to reach US$ 41.0 Bn by 2036, expanding at a compound annual growth rate (CAGR) of 5.3% from 2026 to 2036. This steady growth trajectory reflects the structural importance of gas compression systems across upstream, midstream, and downstream gas value chains. Rising natural gas consumption, expansion of pipeline and LNG infrastructure, and national energy…
Anesthesia Drugs Market to be Worth USD 12.6 Bn by 2036 - By Drug / By Applicati …
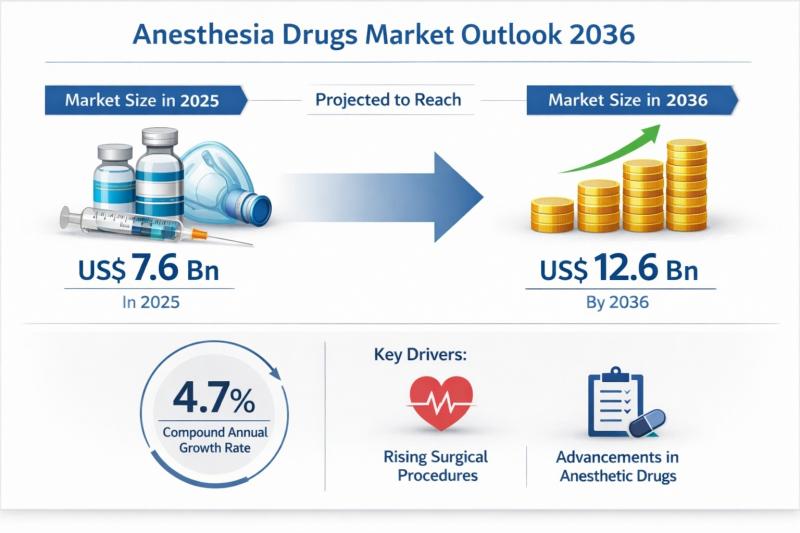
The global anesthesia drugs market was valued at US$ 7.6 billion in 2025 and is projected to reach US$ 12.6 billion by 2036, expanding at a compound annual growth rate (CAGR) of 4.7% from 2026 to 2036. This steady growth trajectory reflects the essential and non-substitutable role of anesthesia drugs in modern healthcare systems. As surgical interventions continue to rise globally-across both elective and emergency procedures-the demand for safe, effective,…
Single-Atom Catalysts Market Size is Expected to Expand from US$ 177.8 Million t …
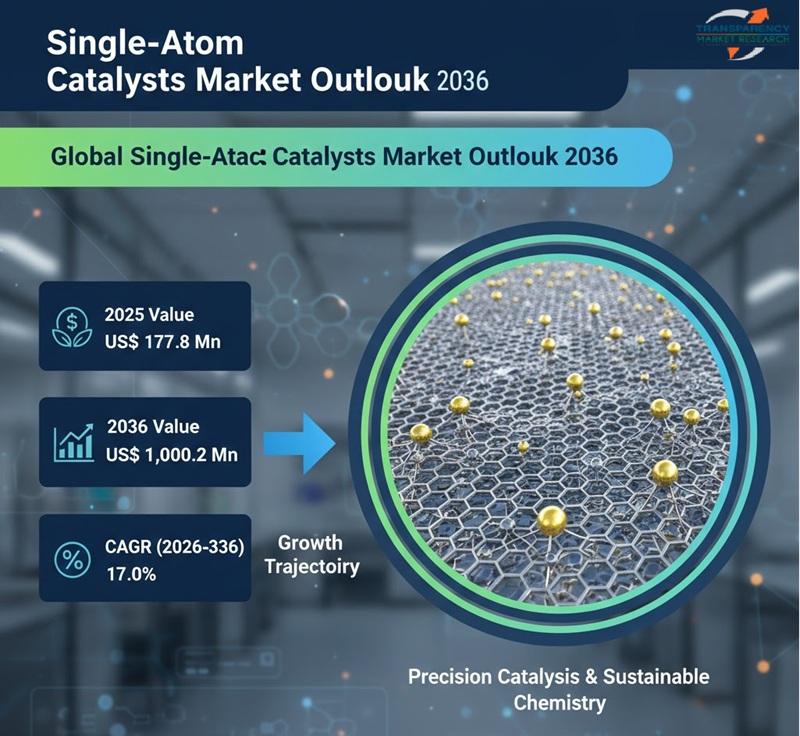
The global single-atom catalysts (SACs) market is poised for remarkable growth as industries seek highly efficient, cost-effective, and sustainable catalytic solutions. Valued at US$ 177.8 million in 2025, the market is projected to reach US$ 1,000.2 million by 2036, expanding at a robust compound annual growth rate (CAGR) of 17.0% from 2026 to 2036. This rapid expansion reflects the growing importance of advanced catalysis in energy, chemicals, environmental protection, and…
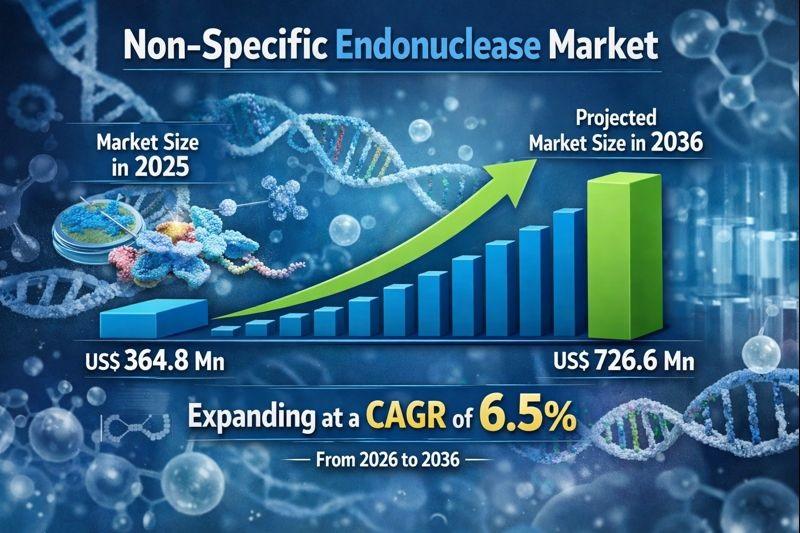
Non-specific Endonuclease Market to Reach USD 726.6 Million by 2036, Supported b …
The non-specific endonuclease market is witnessing steady growth, driven by the expanding use of molecular biology tools across biotechnology, pharmaceuticals, diagnostics, and academic research. Non-specific endonucleases are enzymes that cleave nucleic acids without requiring a specific recognition sequence, making them highly valuable for applications such as DNA/RNA degradation, sample preparation, viscosity reduction, and contamination control. Their broad activity profile differentiates them from restriction enzymes and enables versatile usage across multiple…
More Releases for Inhibitor
Advances in KRAS Inhibitor Development
The development of KRAS inhibitors has been a significant advancement in the field of oncology, offering new hope for patients with cancers driven by KRAS mutations. KRAS, a key gene involved in cell signaling, is frequently mutated in various cancers, including lung, colorectal, and pancreatic cancers. These mutations lead to uncontrolled cell growth and division, making KRAS a critical target for cancer therapy.
Download Report:
https://www.kuickresearch.com/report-kras-inhibitors-market-lumakras-sales-kras-drugs-market-kras-market-size
Historically, KRAS was considered an undruggable target…
Breakthroughs in KRAS Inhibitor Research
KRAS mutations have posed a significant challenge in oncology for decades, but recent breakthroughs in KRAS inhibitor research have sparked hope for new cancer therapies. KRAS is a key regulator of cell growth and survival, and its mutations are prevalent in various cancers, particularly lung, colorectal, and pancreatic cancers. Despite being one of the most sought-after targets, KRAS has been notoriously difficult to inhibit, earning the reputation of being "undruggable."
Download…
Checkpoint Inhibitor Refractory Cancer Market
Checkpoint Inhibitor Refractory Cancer Market Overview
The global Checkpoint Inhibitor Refractory Cancer Market is poised for significant growth, anticipating a high CAGR during the forecast period from 2023 to 2030.
Understanding Checkpoint Inhibitor Refractory Cancer
Refractory cancer, which might not respond to treatments immediately or might develop resistance later, has driven significant growth and transformation in the global checkpoint inhibitor refractory cancer market. The interest of oncologists in the potential antitumor activity of…
Fibroblast Growth Factor Receptor Inhibitor Market - Maximizing Therapeutic Pote …
Newark, New Castle, USA: The "Fibroblast Growth Factor Receptor Inhibitor Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Fibroblast Growth Factor Receptor Inhibitor Market: https://www.growthplusreports.com/report/fibroblast-growth-factor-receptor-inhibitor-market/8933
This latest report…
Global Phosphodiesterase type 5 inhibitor (PDE5 inhibitor) Market Forecast 2023- …
The global Phosphodiesterase type 5 inhibitor (PDE5 inhibitor) market research report helps target customers understand the important driving factors and future opportunities of the industry. The report includes the dynamics of the macro environment such as the latest situation of the war between Russia and Ukraine, the update of monetary policy, and the impact of inflation on the market. Apart from this, the report studies key market developments such as…
Avionic and electronic component corrosion inhibitor
Super CORR A was originally developed for the U.S. Air Force to comply with Mil-DTL-87177B (formerly Mil-L-87177A) specifications. A water displacing lubricant and corrosion protection compound that prevents avionic as well as electrical and electronic components from systems failures caused by corrosion. It is now the industry standard for electrical corrosion protection, for in service maintenance by both military and commercial aircraft major repair and overhaul (MRO) facilities worldwide.
Super CORR…