Press release
Recombinant Cell Culture Supplements Market Persistence Market Research Forecasts 6.1% CAGR Through 2032
The recombinant cell culture supplements market has emerged as a vital component of modern biomanufacturing, enabling high-purity, scalable, and ethically sourced cell culture systems. As biopharmaceutical production increasingly shifts toward serum-free and animal-free media, recombinant supplements have become essential for maintaining consistency, purity, and safety. According to industry projections, the global market is expected to reach US$ 591.3 million by 2025, and continue expanding at a 6.1% CAGR, ultimately achieving US$ 893.4 million by 2032. This strong upward trajectory is fueled by unprecedented growth in biologics, rising chronic disease prevalence, and the continual push toward clean, contamination-free cell culture technologies.Key market trends reflect a growing dependence on recombinant growth factors, cytokines, and proteins that support cell line stability, productivity, and regulatory compliance. Among all market segments, recombinant growth factors are poised to lead with a 25.2% share in 2025, largely due to their critical role in maintaining cell viability in advanced bioprocessing systems. Regionally, North America dominates with a projected 37.4% share in 2025, supported by the presence of major biopharma companies and a strict regulatory environment that encourages the adoption of serum-free and animal-free cell culture media.
Get a Sample Copy of Research Report (Use Corporate Mail id for Quick Response): https://www.persistencemarketresearch.com/samples/31310
Key Highlights from the Report
• Innovations in 3D cell culture and organ-on-a-chip technologies are accelerating the adoption of recombinant supplements.
• Automated and single-use bioreactor systems are increasing the need for consistent, scalable recombinant inputs.
• Regulatory bodies worldwide are strengthening restrictions on animal-derived ingredients in biomanufacturing.
• Recombinant supplements offer an ethical, contamination-free alternative aligned with current compliance standards.
• Manufacturers are launching highly customized recombinant proteins tailored to different cell lines and processes.
• Growth factors lead the product segment due to rising demand for high-quality, stable cell cultures.
Market Segmentation Analysis
The recombinant cell culture supplements market is broadly segmented by product type, application area, and end-user category, each reflecting the diverse and evolving demands of the life sciences industry. Product segmentation is dominated by recombinant growth factors, cytokines, hormones, and proteins that serve as essential components in serum-free media. Growth factors, in particular, hold the largest share due to their ability to stimulate cellular proliferation and maintain viability during complex biomanufacturing processes. Cytokines and recombinant hormones also represent critical product classes, especially for immune cell therapies and stem cell expansion.
In terms of application-based segmentation, stem cell therapy represents the leading segment, projected to hold 30.6% in 2025. This growth is driven by extensive government funding and increasing clinical trial activities centered on regenerative medicine and personalized therapy. Other prominent applications include vaccine development, monoclonal antibody production, cell-based research, and tissue engineering. As biologics advance, demand for specialized recombinant supplements tailored to specific cell lines continues to rise.
The end-user landscape features biopharmaceutical manufacturers, academic research institutions, cell therapy developers, and contract manufacturing organizations (CMOs). Biopharma companies remain the primary consumers, using recombinant supplements to optimize large-scale production of vaccines, CAR-T therapies, monoclonal antibodies, and advanced therapeutics. CMOs and CROs are also expanding recombinant supplement usage as they shift from small-scale to high-throughput, GMP-compliant production environments.
Read Detailed Analysis: https://www.persistencemarketresearch.com/market-research/recombinant-cell-culture-supplements-market.asp
Regional Insights
Regional dynamics in the recombinant cell culture supplements market reveal distinct patterns shaped by regulatory environments, technological advancement, and investment trends. North America stands as the dominant region, capturing a projected 37.4% of the market in 2025. Its leadership is reinforced by major industry players, strong R&D investments, and strict FDA guidelines promoting serum-free, animal-free cell culture systems. The U.S. remains the epicenter for cell and gene therapy development, with 20 new FDA approvals in 2023 alone, significantly elevating demand for recombinant growth factors and cytokines.
Europe follows closely, driven by ethical considerations surrounding animal-derived components and stringent regulations such as the EU Directive 2010/63/EU. European biopharmaceutical companies are increasing their dependence on recombinant technologies to reduce variability and ensure regulatory compliance. Germany, the U.K., and Switzerland remain strongholds for innovation in recombinant media systems.
Asia-Pacific is emerging as a high-growth region fueled by expanding biologics manufacturing capabilities, rising government support for stem cell research, and increasing investment in domestic biopharma industries. Countries such as China, Japan, and South Korea are accelerating their adoption of recombinant supplements as they expand their vaccine production infrastructure and cell therapy pipelines. With growing research funding and new facility expansions, APAC is expected to show one of the highest growth rates through 2032.
Regions such as Latin America and the Middle East & Africa are gradually adopting recombinant supplements in tandem with rising biotechnology investments and increasing demand for cell-based research. These markets remain in early stages but are expected to benefit from global outsourcing trends and capacity expansions by international manufacturers.
Market Drivers
One of the most significant drivers of the recombinant cell culture supplements market is the transition toward serum-free and animal-free culture systems. Traditional fetal bovine serum (FBS) poses risks of viral, prion, and mycoplasma contamination, creating major complications for biopharmaceutical manufacturers. Recombinant supplements eliminate these risks, offering high-purity, consistent formulations that align with GMP requirements. With adoption of serum-free systems increasing by 25% between 2019 and 2023, the shift reflects both ethical considerations and the demand for reproducibility in large-scale biologics production.
Another powerful driver is the global expansion of vaccine development and biopharmaceutical research. The rise in infectious diseases, the emergence of new viral strains, and the unprecedented scale of vaccine production witnessed during the COVID-19 pandemic have amplified the need for recombinant supplements. Modern vaccines, including mRNA-based and viral vector vaccines, require highly controlled environments and consistent media components to achieve optimal yields. Recombinant proteins and cytokines enable scalable production and improved safety profiles, making them indispensable to vaccine manufacturers worldwide.
Market Restraints
Despite strong growth, the market faces notable restraints related to contamination risks and the high sensitivity of recombinant production systems. Cell culture environments are vulnerable to microbial, chemical, and cross-contamination, which can compromise entire batches and lead to substantial financial losses. Mycoplasma, in particular, represents up to 50% of contamination incidents, underscoring the need for stringent monitoring and quality control. Even minor contamination can result in inaccurate research results or halted production, discouraging smaller facilities with limited resources from adopting recombinant technologies.
The complexity and cost of producing recombinant supplements also constrain broader adoption. Recombinant protein expression requires skilled labor, advanced bioprocessing infrastructure, and rigorous purification systems to meet regulatory standards. This increases the final product cost, posing challenges for academic labs, small biotech startups, and emerging market manufacturers. Variability in production methods and the technical challenges associated with scaling further contribute to adoption barriers.
Market Opportunities
The recombinant cell culture supplements market is entering a transformative period fueled by opportunities in tissue engineering, regenerative medicine, and next-generation bioprocessing. The global shortage of transplantable organs-where only 10% of demand was met in 2022-has accelerated the need for engineered tissues and artificial organs. Recombinant supplements offer the consistency and purity required to develop functional tissue constructs. Their role in supporting precise cellular environments makes them indispensable in producing engineered tissues for clinical and research applications.
Advancements in recombinant technology represent another major opportunity. Breakthroughs in CRISPR-Cas9 gene editing, mammalian and yeast expression systems, and automated bioprocessing are significantly reducing production costs and variability. Mammalian cell systems now account for 70% of recombinant protein production, demonstrating their dominance in generating bioactive, fully functional recombinant supplements. Continuous manufacturing, improved expression platforms, and scalable purification technologies are enabling greater accessibility, particularly for institutions transitioning to GMP-compliant workflows. As automation adoption surpasses 40% in biomanufacturing facilities, the market is poised for rapid expansion in both capacity and innovation.
Request for Customization of the Research Report: https://www.persistencemarketresearch.com/request-customization/31310
Company Insights
• Merck KGaA
• Thermo Fisher Scientific Inc.
• GE Healthcare
• Lonza Group AG
• F. Hoffmann-La Roche
• Abcam PLC
• Hi-Media Laboratories
• Becton, Dickinson and Company
• STEMCELL Technologies Inc.
• Fujifilm Corporation
• InVitria
• Biocon
• Cell Sciences, Inc.
• Corning Incorporated
• Sartorius AG
• Repligen Corporation
• Bio-Techne Corporation
• Miltenyi Biotec
Market Segmentation
By Product
Recombinant Growth Factors
Transforming Growth Factor
Epidermal Growth Factor
Platelet-Derived Growth Factors
Fibroblast Growth Factor
Vascular Endothelial Growth Factors (VEGFs)
Interleukins
Recombinant Stem Cell Factor
Other
Recombinant Insulin
Recombinant Albumin
Recombinant Transferrin
Recombinant Trypsin
Recombinant Aprotinin
Recombinant Lysozyme
Others
By Source
Animals
Microorganisms
Human
By Application
Stem Cell Therapy
Gene Therapy
Bioprocess Application
Vaccine Development
Others
By End User
Academic and Research Institutes
Biopharmaceutical Companies
Cancer Research Centers
Contract Research Centers (CROs)
By Region
North America
Latin America
Europe
East Asia
South Asia and Oceania
Middle East and Africa
Recent Developments
Future Fields launched the world's first fruit-fly-based synthetic biology system in January 2024, debuting its Recombinant Human Prolactin (PRL-Fc) to advance cost-effective protein production.
FUJIFILM Irvine Scientific expanded its BalanCD CHO Media Platform in April 2023 with two new chemically defined media to improve CHO cell growth and biotherapeutic yields.
Lonza released the TheraPEAK® T-VIVO® Cell Culture Medium in May 2023, an animal-free, chemically defined medium optimized for scalable CAR-T cell manufacturing.
Conclusion
The recombinant cell culture supplements market is experiencing a period of robust growth driven by the shift toward serum-free, animal-free production systems, advancements in biopharmaceutical manufacturing, and an expanding global focus on regenerative medicine. As the biopharma sector continues its shift toward precision therapies, cell-based technologies, and next-generation vaccines, recombinant supplements will remain foundational for supporting innovation and scalability. While challenges related to contamination risks and production complexities persist, emerging technological breakthroughs and increasing regulatory support are paving the way for widespread adoption. With rising demand for biologics, expanding stem cell research funding, and heightened attention to ethical sourcing, the market is positioned to evolve rapidly, offering substantial opportunities for manufacturers, researchers, and healthcare innovators worldwide.
Read More Related Reports:
Oral Hygiene Products Market https://www.persistencemarketresearch.com/market-research/oral-hygiene-products-market.asp
Norovirus Diagnostics Market https://www.persistencemarketresearch.com/market-research/norovirus-infection-diagnostics-market.asp
Injectable Nanomedicines Market https://www.persistencemarketresearch.com/market-research/injectable-nanomedicines-market.asp
Cosmetic Peptide Synthesis Market https://www.persistencemarketresearch.com/market-research/cosmetic-peptide-synthesis-market.asp
Contact Us:
Persistence Market Research
Second Floor, 150 Fleet Street, London, EC4A 2DQ, United Kingdom
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Recombinant Cell Culture Supplements Market Persistence Market Research Forecasts 6.1% CAGR Through 2032 here
News-ID: 4274676 • Views: …
More Releases from Persistence Market Research

Nickel-Metal Hydride Battery Market Predicted to See Expansion to US$ 4.9 Billio …
According to the latest study by Persistence Market Research, the global Nickel-Metal Hydride Battery Market is expected to be valued at US$ 3.6 billion in 2026 and is projected to reach US$ 4.9 billion by 2033, growing at a CAGR of 4.5% between 2026 and 2033. Despite the rapid rise of lithium-ion technologies, NiMH batteries continue to maintain strong relevance due to their safety profile, long cycle life, and established…
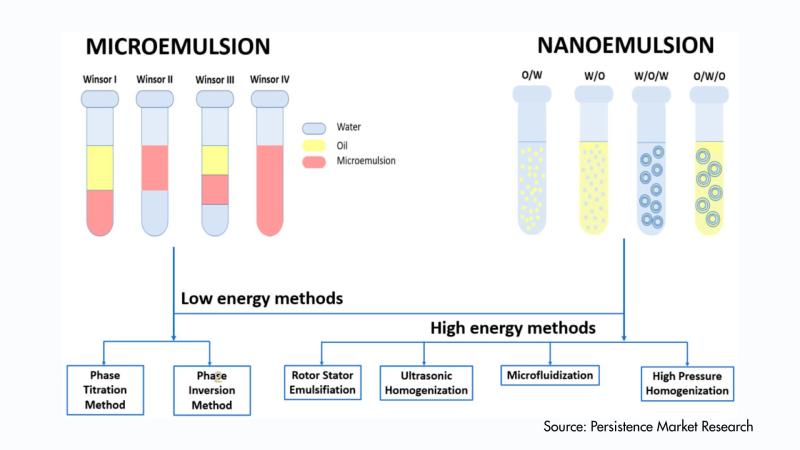
Microemulsion Market Poised for Growth to US$ 2.8 Billion by 2033, Driven by Exp …
According to the latest study by Persistence Market Research, the global microemulsion market is likely to be valued at US$ 1.8 billion in 2026 and is expected to reach US$ 2.8 billion by 2033, growing at a CAGR of 6.5% during the forecast period from 2026 to 2033. This steady growth reflects the rising demand for advanced formulation technologies across multiple industries, including pharmaceuticals, personal care, food & beverages, and…
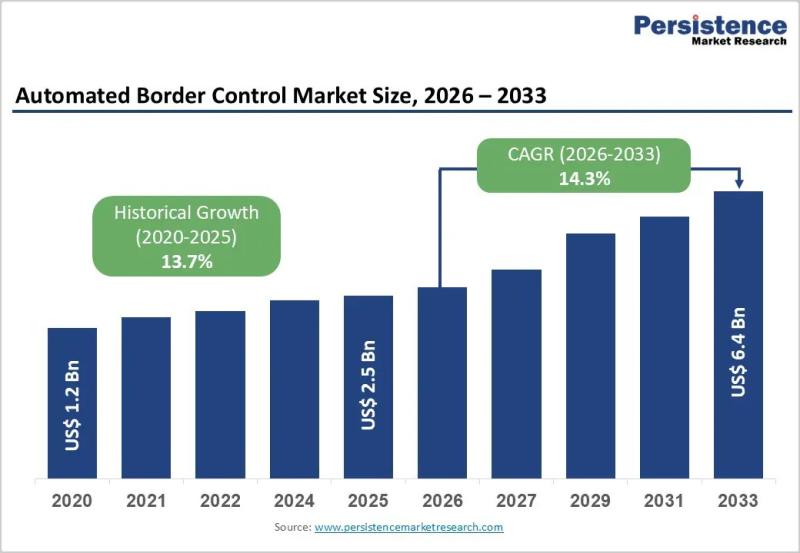
Automated Border Control Market Accelerates as Smart Borders Redefine Global Tra …
The automated border control (ABC) market has become a critical pillar of modern border management as governments worldwide respond to rising international travel volumes, security challenges, and the need for faster passenger processing. Automated border control systems use advanced biometric technologies-such as facial recognition, fingerprint scanning, and iris recognition-to verify traveler identities with minimal human intervention. These systems are most commonly deployed through ABC e-gates at airports, but their adoption…

Aluminum Composite Panels Market Set to Grow to US$14.0 Billion by 2033 Driven b …
The Aluminum Composite Panels Market is witnessing steady expansion as global construction activity accelerates and demand rises for lightweight, durable, and visually appealing building materials. According to the latest study by Persistence Market Research, the global aluminum composite panels market size is likely to be valued at US$ 9.1 billion in 2026 and is expected to reach US$ 14.0 billion by 2033, growing at a CAGR of 6.3% during the…
More Releases for Recombinant
Recombinant Human Endostatin Market Experiences Surge Due to Innovations in Reco …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Recombinant Human Endostatin Market - (By Type (purity 95% and others) And By Application (medical care, scientific research and others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global Recombinant Human Endostatin Market is valued at US$ 133.3 Mn in 2023, and it is expected to…
Recombinant Chemicals Market Expands with Growing Adoption of Recombinant DNA an …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Recombinant Chemicals Market - (By Product Type (Recombinant Proteins, Recombinant DNAs, Recombinant Peptides, Others), By Technology (Microbial Expression Systems, Mammalian Cell Expression Systems, Yeast Expression Systems, Insect Cell Expression Systems, Cell-free Expression Systems), By Application (Drug Development, Vaccine Production, Therapeutics, Cell Line Development, Agriculture, Food and Beverage, Others)), Trends, Industry Competition Analysis, Revenue and Forecast…
Recombinant Collagen Market Size and Forecast
Recombinant Collagen Market size was valued at USD 0.9 Billion in 2022 and is projected to reach USD 1.8 Billion by 2030, growing at a CAGR of 9.0% from 2024 to 2030.
Recombinant Collagen Market Outlook and Investment Analysis
Top companies
Giant Biogene Holding, Avantor, ProColl, Merck, ACROBiosystems, Wuhan Fine Biotech, Shanxi Jinbo Bio-Pharmaceutical, Jiangsu Jland Biotech, Jiangsu Trautec Medical Technology, OriGene Technologies
Recombinant Collagen Market: Trends & Investment Analysis
Market Growth: The recombinant collagen…
Global Recombinant Cell Culture Supplements Market Size - By Product Type(Recomb …
Recombinant Cell Culture Supplements Market Insights: Trends, Drivers, and Outlook 2024 - 2031
Recombinant Cell Culture Supplements Market Scope: Unveiling Today's Trends
Recombinant Cell Culture Supplements are specialized products designed to enhance growth and productivity in cells used for research and bioproduction.
The market for these supplements is experiencing substantial growth, driven by the rising demand for biopharmaceuticals and advancements in biotechnology. Increasing investments in research and development activities also contribute to market…
Recombinant Protein Market Size, Share | Recombinant Protein Industry Future Gro …
Global Recombinant Protein Market Report provides a detailed industry overview along with the analysis of Cost Structure, Supply Chain, Development Techniques, Retailers Analysis, Financial Support, business Strategies, Marketing Channels. Global Recombinant Protein Market research report provides a point-by-pointIn-Depth analysis of global market size, regional and country-level market size, segmentation market growth, market share, competitive Landscape, sales analysis, the impact of domestic and global market players, value chain optimization, trade regulations,…
Recombinant Proteins Market 2022 | Recombinant Proteins Market: Global Industry …
Recombinant proteins play an imperative role in treating various diseases, such as hemophilia. Majority of the recombinant proteins used are human proteins, in order to compensate the functional proteins in vivo defects by increasing the protein function in a body. This Research Report Insights analyzes the expansion of global recombinant protein market till date, and provides key insights on the growth of the market during the forecast period, 2017-2022.
The next…
