Press release
Myasthenia Gravis Market is expected to grow at a CAGR of 10.4% by 2034, estimates DelveInsight
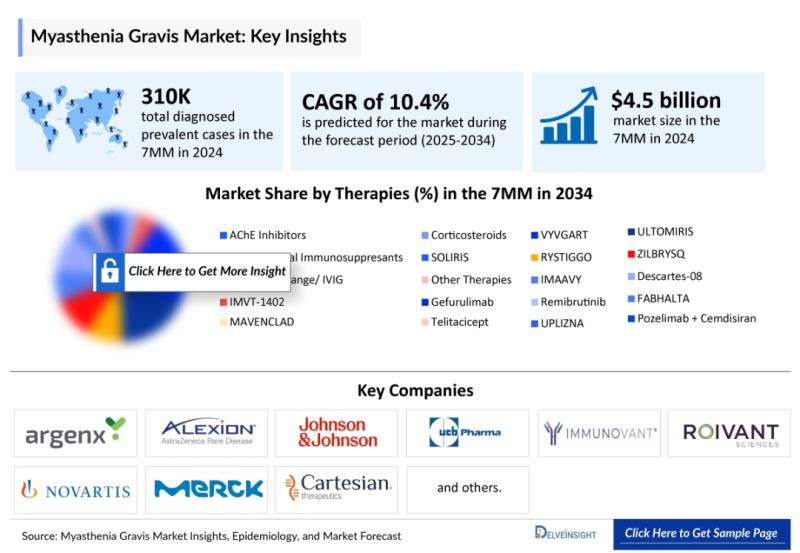
Myasthenia Gravis companies such as Hoffmann-La Roche, Immunovant Sciences GmbH, Horizon Therapeutics, Amgen, Janssen Research & Development, Alexion, AstraZeneca Rare Disease, Regeneron Pharmaceuticals, Kyverna Therapeutics, Cartesian Therapeutics, Dianthus Therapeutics, Takeda, COUR Pharmaceuticals, and others.The myasthenia gravis (MG) market, valued at approximately USD 4.5 billion in 2024, is projected to expand at a robust CAGR of 10.4% through 2034. In 2024, the prevalence across the seven major markets (7MM) reached an estimated 310,000 diagnosed cases, with the United States representing ~137,000 cases, showing a marginal predominance in males. The treatment landscape is rapidly evolving toward precision immunomodulation, propelled by the adoption of targeted biologics such as FcRn inhibitors (RYSTIGGO, VYVGART, IMAAVY) and complement pathway inhibitors (SOLIRIS, ULTOMIRIS, ZILBRYSQ).
A new wave of investigational therapies including RNA-based and CAR-T-like modalities, dual complement inhibitors, and BLyS/APRIL axis blockers seeks to re-establish durable immune homeostasis rather than provide transient symptomatic control. Despite these advances, substantial unmet needs persist, particularly for rapid-onset, durable, and safer treatments, as many patients continue to experience delayed therapeutic response, relapse, or treatment-associated toxicities.
DelveInsight's Myasthenia Gravis Market Insights report offers an in-depth overview of current treatment approaches, upcoming therapies, individual drug market shares, and both historical and projected market size for myasthenia gravis from 2020 to 2034 across the 7MM, which includes the United States, EU4 (Italy, Spain, France, and Germany), the United Kingdom, and Japan. The myasthenia gravis market is expected to evolve significantly in the coming years, driven by advancements in diagnostic techniques, growing disease awareness, increased global healthcare expenditure, and the anticipated introduction of novel therapies.
Discover which therapies are expected to grab the major myasthenia gravis market share @ [https://www.delveinsight.com/report-store/myasthenia-gravis-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Key Takeaways from the Myasthenia Gravis Market Report
* As per DelveInsight's analysis, the myasthenia gravis market is anticipated to grow at a significant CAGR by 2034.
* The Myasthenia Gravis market's total size in the 7MM reached approximately USD 4500 million in 2024. Projections indicate a substantial growth during the forecast period.
* The overall count of individuals diagnosed with myasthenia gravis in the United States was approximately 129 thousand in 2023, and it is expected to increase at an estimated CAGR throughout the study period (2020-2034).
* Leading myasthenia gravis companies such as Horizon Therapeutics, Amgen, Janssen Research & Development, LLC, Hoffmann-La Roche, Immunovant Sciences GmbH, Alexion, AstraZeneca Rare Disease, Regeneron Pharmaceuticals, Kyverna Therapeutics, Cartesian Therapeutics, Dianthus Therapeutics, Takeda, COUR Pharmaceuticals, and others are developing novel myasthenia gravis drugs that can be available in the myasthenia gravis market in the coming years.
* Some of the key therapies for myasthenia gravis treatment include DAS-001, Amifampridine Phosphate, MuSK-CAART, Nipocalimab, Efgartigimod IV, Rozanolixizumab, ALXN1720, Batoclimab, Descartes-08, Zilucoplan (RA101495), and others.
* In October 2025, Johnson & Johnson (NYSE: JNJ) today announced plans to initiate the first head-to-head study comparing FcRn blockers for patients with generalized myasthenia gravis (gMG), which aims to affirm IMAAVY Trademark (nipocalimab-aahu) as the FcRn blocker of choice for appropriate gMG patients. The EPIC study design and Vibrance-MG Phase 2/3 long-term extension (LTE) data are among 38 abstracts the Company will present at the 2025 Myasthenia Gravis Foundation of America (MGFA) Scientific Session and the American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM) Annual Meeting.
* In October 2025, COUR Pharma, a clinical-stage biotechnology company developing rst-in-class, antigen-specific immune tolerance therapies for autoimmune diseases, announced today the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to CNP-106, an investigational therapy for the treatment of generalized myasthenia gravis (gMG)
* In August 2025, Kyverna Therapeutics, Inc. (Nasdaq: KYTX), a clinical-stage biopharmaceutical company focused on developing cell therapies for patients with autoimmune diseases, will host a virtual KOL event today to highlight its neuroimmunology CAR T franchise in stiff person syndrome (SPS) and myasthenia gravis (MG).
* In April 2025, Impressive findings from the Phase 3 clinical trial (NCT05737160) evaluating the safety and effectiveness of Telitacicept (also known as RC18; brand name: Registered ) in individuals with generalized myasthenia gravis (gMG) were presented during the Late-Breaking Science Session at the 2025 American Academy of Neurology (AAN) Annual Meeting.
* In March 2025, Immunovant has opted not to seek regulatory approval for its myasthenia gravis (MG) therapy, batoclimab, even though the Phase III trial achieved its primary goal of reducing disease symptoms. Instead, the company plans to use the trial data to support the advancement of its alternative candidate, IMVT-1402. The Phase III randomized study (NCT05403541) evaluated the effects of weekly or bi-weekly batoclimab dosing in acetylcholine receptor antibody-positive (AChR+) patients, measuring improvements through the Myasthenia Gravis Activities of Daily Living (MG-ADL) score over 12 weeks.
* In March 2025, The Muscular Dystrophy Association (MDA) welcomes the U.S. Food and Drug Administration (FDA) approval of the expanded indication of Alexion/AstraZeneca's eculizumab (Soliris) for pediatric patients aged six years and older with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive. This landmark approval makes Soliris the first and only treatment available for pediatric patients living with this debilitating neuromuscular disease.
* In January 2025, Cartesian Therapeutics has secured an agreement from the US Food and Drug Administration (FDA) under the Special Protocol Assessment (SPA) process for its phase 3 AURORA trial of Descartes-08, an mRNA cell therapy candidate for myasthenia gravis (MG).
* In December 2024, Alexion Pharmaceuticals, Inc. an Open-Label, Multicenter Study to Evaluate the Efficacy, Safety, Pharmacokinetics, and Pharmacodynamics of Eculizumab in Pediatric Patients With Refractory Generalized Myasthenia Gravis
* In October 2023, UCB S.A., a global leader in biopharmaceuticals, announced that ZILBRYSQ (zilucoplan) received U.S. FDA approval for treating generalized myasthenia gravis (gMG) in adults who are anti-acetylcholine receptor (AChR) antibody-positive. This approval marks a significant advancement in the treatment of gMG, offering a targeted therapy for patients who previously had limited options
* In September 2023, Recipharm AB announced a strategic partnership with AHEAD THERAPEUTICS S.L. to advance the development of a new therapy for myasthenia gravis, a chronic autoimmune disorder. This collaboration focuses on scaling up the manufacturing capabilities for myasthenia gravis treatments, ensuring greater production capacity to meet growing patient needs.
Myasthenia Gravis Overview
Myasthenia gravis (MG) is a chronic autoimmune neuromuscular disorder characterized by fluctuating muscle weakness and fatigability caused by impaired transmission at the neuromuscular junction. The condition most commonly results from autoantibodies targeting acetylcholine receptors (AChR) or muscle-specific kinase (MuSK), which disrupt synaptic signaling and reduce muscle activation. Symptoms typically include ptosis, diplopia, dysphagia, limb weakness, and respiratory compromise in severe cases. MG can occur at any age, with early-onset disease often linked to thymic abnormalities and late-onset disease more common in older adults. Diagnosis involves clinical assessment, serological antibody testing, electrophysiological studies, and imaging of the thymus. Treatment strategies include acetylcholinesterase inhibitors, corticosteroids, immunosuppressants, and biologic therapies such as complement inhibitors and FcRn blockers that target key immune pathways. Thymectomy remains an important option for select patients. Advances in precision immunotherapy continue to improve disease control, reduce exacerbations, and enhance long-term outcomes.
Myasthenia Gravis Epidemiology
In 2024, the US recorded approximately 137,000 diagnosed myasthenia gravis cases, a number expected to rise by 2034 with better disease awareness and diagnostics. Across EU4 and the UK, Germany reported the highest prevalence at around 37,000 cases, while Spain had the lowest with about 14,000. In Germany, females made up 52% of diagnosed cases, reflecting a slight but consistent gender dominance. Regarding disease severity across EU4 and the UK, mild generalized MG (Class II) was the most common, affecting around 50,000 individuals, followed by ocular MG (Class I) at over 45,000 and moderate generalized (Class III) at more than 28,000. Severe cases (Class IV and V) remained comparatively rare, totaling under 10,000, emphasizing that most patients have milder disease.
In Japan, anti-AChR-positive patients formed the majority of gMG cases (~19,000), while anti-MuSK and double-seronegative groups accounted for smaller proportions.
Myasthenia Gravis Epidemiology Segmentation
The myasthenia gravis epidemiology section provides insights into the historical and current myasthenia gravis patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders.
The myasthenia gravis market report proffers epidemiological analysis for the study period 2020-2034 in the 7MM segmented into:
* Total Generalized Myasthenia Gravis Diagnosed Prevalent Cases
* Generalized Myasthenia Gravis Gender-Specific Cases
* Generalized Myasthenia Gravis Antibodies-Specific Cases
Download the report to understand which factors are driving myasthenia gravis epidemiology trends @ [https://www.delveinsight.com/sample-request/myasthenia-gravis-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Myasthenia Gravis Treatment Market
The treatment of myasthenia gravis typically involves a multi-faceted approach aimed at managing its symptoms and improving the patient's quality of life. The cornerstone of myasthenia gravis treatment is the use of medications, most commonly acetylcholinesterase inhibitors like pyridostigmine, which enhance communication between nerve and muscle cells. Immunosuppressive drugs, such as corticosteroids, azathioprine, or mycophenolate mofetil, may also be prescribed to reduce the immune system's attack on the neuromuscular junction.
In more severe cases or during myasthenia gravis crises, where symptoms become life-threatening, treatments like intravenous immunoglobulin (IVIG) or plasmapheresis may be employed to rapidly suppress the immune response. Additionally, some patients benefit from surgical interventions, such as thymectomy, to remove the thymus gland, which is often implicated in myasthenia gravis. It's essential for myasthenia gravis patients to work closely with healthcare professionals to tailor their treatment plans to their specific needs, as responses to medications and therapies can vary significantly among individuals. Regular follow-up appointments and adjustments to treatment are typically necessary to effectively manage this chronic autoimmune disorder.
Learn more about the FDA-approved drugs for myasthenia gravis @ [https://www.delveinsight.com/report-store/myasthenia-gravis-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Myasthenia Gravis Competitive Landscape
* Tolebrutinib: Sanofi
* Descartes-08: Cartesian Therapeutics
* Mezagitamab (TAK-079): Takeda
* DAS-001: DAS Therapeutics, Inc.
* Rozanolixizumab: UCB Biopharma
* Zilucoplan: UCB Biopharma
* Subcutaneous Efgartigimod: Argenx-Halozyme Therapeutics
* Uplizna (Inebilizumab): Horizon Therapeutics
* Enspryng (Satralizumab): Hoffmann-La Roche
* Nipocalimab: Janssen Research & Development, LLC
* Batoclimab: Immunovant Sciences GmbH
To know more about clinical trials, visit @ [https://www.delveinsight.com/sample-request/myasthenia-gravis-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Myasthenia Gravis Market Dynamics
Myasthenia Gravis Market Drivers
* Rising Disease Prevalence & Improved Diagnosis
Growing awareness and advancements in diagnostic tools are leading to earlier and more accurate detection of MG, expanding the treatable patient population.
* Shift Toward Precision & Targeted Therapies
Innovations such as FcRn inhibitors (e.g., VYVGART, RYSTIGGO) and complement inhibitors (e.g., SOLIRIS, ULTOMIRIS, ZILBRYSQ) are transforming treatment paradigms, driving adoption of premium-priced therapies.
* Strong R&D Pipeline
Emerging candidates like RNA CAR T-cell therapies, dual complement inhibitors, and BLyS/APRIL blockers indicate sustained innovation and future market expansion.
* Increasing Healthcare Expenditure & Reimbursement Support
Growing insurance coverage for high-cost biologics in major markets supports accessibility and uptake.
* Shift From Symptom Control to Long-Term Immune Modulation
A global focus on durable disease control rather than episodic management supports higher demand for maintenance therapies.
Myasthenia Gravis Market Barriers
* High Cost of Biologic and Advanced Therapies
Premium pricing limits accessibility, especially in regions with weaker reimbursement frameworks.
* Delayed Onset of Action & Relapse Risks
Many therapies still fail to provide rapid or sustained relief, leaving unmet needs despite treatment.
* Heterogeneous Disease Presentation
Variability in antibody subtypes (AChR+, MuSK+, seronegative) complicates treatment selection and standardization.
* Limited Long-Term Safety Data for Emerging Therapies
Physicians may hesitate to adopt newer agents without established risk profiles.
* Dependence on Specialist Care & Infusion-Based Therapies
Complex administration requirements restrict treatment to specialized centers, affecting penetration in remote areas.
Scope of Myasthenia Gravis Market Report:
* Study Period: 2020-2034
* Myasthenia Gravis Report Coverage: 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan]
* Key Myasthenia Gravis Companies: DAS-MG, Inc, Catalyst Pharmaceuticals, Inc., Cabaletta Bio, Janssen Research & Development, LLC, Argenx, UCB Pharma, Alexion, Immunovant Sciences GmbH, Cartesian Therapeutics, Ra Pharmaceuticals, Inc., and others
* Key Myasthenia Gravis Therapies: DAS-001, Amifampridine Phosphate, MuSK-CAART, Nipocalimab, Efgartigimod IV, Rozanolixizumab, ALXN1720, Batoclimab, Descartes-08, Zilucoplan (RA101495), and others
* Therapeutic Assessment: Myasthenia Gravis current marketed and emerging therapies
* Myasthenia Gravis Market Dynamics: Attribute Analysis of Emerging Myasthenia Gravis Drugs
* Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
* Unmet Needs, KOL's views, Analyst's views, Myasthenia Gravis Market Access and Reimbursement
Discover more about myasthenia gravis drugs in development @ [https://www.delveinsight.com/sample-request/myasthenia-gravis-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Table of Contents
1. Myasthenia Gravis Market Key Insights
2. Myasthenia Gravis Market Report Introduction
3. Myasthenia Gravis Market Overview at a Glance
4. Myasthenia Gravis Market Executive Summary
5. Disease Background and Overview
6. Myasthenia Gravis Treatment and Management
7. Myasthenia Gravis Epidemiology and Patient Population
8. Myasthenia Gravis Patient Journey
9. Myasthenia Gravis Marketed Drugs
10. Myasthenia Gravis Emerging Drugs
11. Seven Major Myasthenia Gravis Market Analysis
12. Myasthenia Gravis Market Outlook
13. Potential of Current and Emerging Therapies
14. KOL Views on Myasthenia Gravis
15. Myasthenia Gravis Unmet Needs
16. Myasthenia Gravis SWOT Analysis
17. Appendix
18. DelveInsight Capabilities
19. Disclaimer
20. About DelveInsight
About DelveInsight
DelveInsight is a leading Healthcare Business Consultant, and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance. It also offers Healthcare Consulting Services, which benefits in market analysis to accelerate the business growth and overcome challenges with a practical approach.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=myasthenia-gravis-market-is-expected-to-grow-at-a-cagr-of-104-by-2034-estimates-delveinsight]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/consulting/primary-research-services
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Myasthenia Gravis Market is expected to grow at a CAGR of 10.4% by 2034, estimates DelveInsight here
News-ID: 4271950 • Views: …
More Releases from ABNewswire

How to Check the "Cutting Edge" After a Tungsten Carbide Blades are Made
How to Check the "Cutting Edge" After a Tungsten Carbide blades are Made? We can think of it as :giving a final inspection to the armor and weapons of a general about to go into battle.
I. What Tools or equipment Are Used for Inspection?
1. "Extension of the Eyes" - Optical Magnifiers:
Tools: Bench magnifiers, illuminated magnifiers, stereomicroscopes.
What they're for: This is the most common, first-step inspection. Just like using a magnifying…

How to Check the "Cutting Edge" After a Tungsten Carbide Blades are Made
How to Check the "Cutting Edge" After a Tungsten Carbide blades are Made? We can think of it as :giving a final inspection to the armor and weapons of a general about to go into battle.
I. What Tools or equipment Are Used for Inspection?
1. "Extension of the Eyes" - Optical Magnifiers:
Tools: Bench magnifiers, illuminated magnifiers, stereomicroscopes.
What they're for: This is the most common, first-step inspection. Just like using a magnifying…

How to Check the "Cutting Edge" After a Tungsten Carbide Blades are Made
How to Check the "Cutting Edge" After a Tungsten Carbide blades are Made? We can think of it as :giving a final inspection to the armor and weapons of a general about to go into battle.
I. What Tools or equipment Are Used for Inspection?
1. "Extension of the Eyes" - Optical Magnifiers:
Tools: Bench magnifiers, illuminated magnifiers, stereomicroscopes.
What they're for: This is the most common, first-step inspection. Just like using a magnifying…

How to Check the "Cutting Edge" After a Tungsten Carbide Blades are Made
How to Check the "Cutting Edge" After a Tungsten Carbide blades are Made? We can think of it as :giving a final inspection to the armor and weapons of a general about to go into battle.
I. What Tools or equipment Are Used for Inspection?
1. "Extension of the Eyes" - Optical Magnifiers:
Tools: Bench magnifiers, illuminated magnifiers, stereomicroscopes.
What they're for: This is the most common, first-step inspection. Just like using a magnifying…
More Releases for Myasthenia
Myasthenia Gravis (MG) Treatment Market: Insights and Trends
The Global Myasthenia Gravis (MG) Treatment Market is experiencing notable growth, driven by increasing awareness of the disease and advancements in treatment options. Myasthenia Gravis is a chronic autoimmune disorder that leads to varying degrees of muscle weakness, and effective management is crucial for improving patient quality of life.
With ongoing research and development in treatment modalities, the market is poised for significant expansion over the coming years.
Market Size and Growth:
The…
Myasthenia Gravis Market | Rising Prevalence Of Neuromuscular Disorders
According to Precision Business Insights (PBI), the latest report, the Myasthenia Gravis market is expected to be valued at USD 1,734.6 million in 2022, growing at an 8.1% CAGR from 2022 to 2028. The primary driver of the expansion of the global Myasthenia Gravis market increasing prevalence of neuromuscular disorders, and rising awareness about effective treatment.
View the detailed report description here - https://precisionbusinessinsights.com/market-reports/myasthenia-gravis-market/
Medication Segment to Dominate the Myasthenia…
Myasthenia Gravis - Drug Pipeline Landscape, 2022
Myasthenia gravis is a chronic autoimmune neuromuscular condition that causes muscle weakness and severe fatigue. It causes weakness in the skeletal muscles, which are the muscles your body uses for movement. Myasthenia gravis is caused by a breakdown in the normal communication between nerves and muscles.
Healthcare professional checks neurological health by testing reflexes, muscle strength, muscle tone etc. Ice pack test, antibody tests, imaging scans and electromyogram can also be…
Myasthenia Gravis Industry Report Analysis By opportunities
Stratistics MRC's Myasthenia Gravis Market report provides readers with an understanding of market details, overview, drivers and segmentation with types.
Myasthenia gravis is a chronic autoimmune neuromuscular disorder that is characterized by variable weakness of the voluntary muscle groups. Muscles that observe eye movement, shoulder & facial muscles, breathing & swallowing and eye lids are the ones that are regularly impacted in myasthenia gravis.
Browse complete "Myasthenia Gravis Market" report with TOC…
Myasthenia Gravis Industry Report Analysis By opportunities
Stratistics MRC's Myasthenia Gravis Market report provides readers with an understanding of market details, overview, drivers and segmentation with types.
Myasthenia gravis is a chronic autoimmune neuromuscular disorder that is characterized by variable weakness of the voluntary muscle groups. Muscles that observe eye movement, shoulder & facial muscles, breathing & swallowing and eye lids are the ones that are regularly impacted in myasthenia gravis.
Browse complete "Myasthenia Gravis Market" report with TOC…
Global Myasthenia Gravis Industry Analysis By Geography
Stratistics MRC's Myasthenia Gravis Market report explains company profiling, key segments, market trends, top players and regional, country-level segments.
Myasthenia gravis is a chronic autoimmune neuromuscular disorder that is characterized by variable weakness of the voluntary muscle groups. Muscles that observe eye movement, shoulder & facial muscles, breathing & swallowing and eye lids are the ones that are regularly impacted in myasthenia gravis.
Browse complete "Myasthenia Gravis Market" report with TOC @…