Press release
Comprehensive Study on the Clinical Trial Supplies Market: Growth Drivers and Regional Dynamics | Meticulous Research®
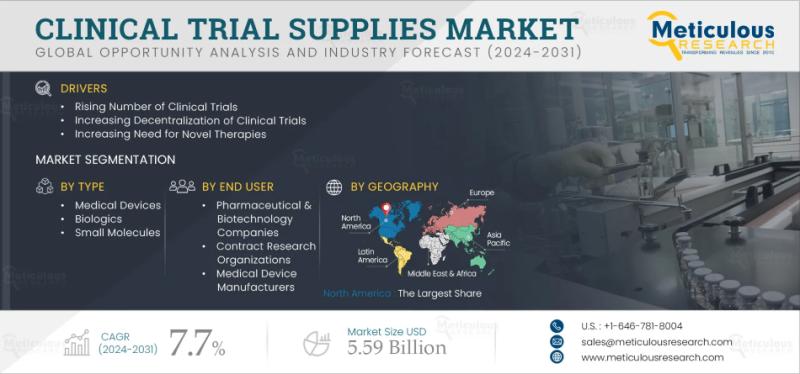
The global market for clinical trial supplies has grown steadily over the past few years. Valued at roughly $3.11 billion in 2023, it is expected to climb to $5.59 billion by 2031, marking a healthy compound annual growth rate of 7.7% from 2024 onward. This growth reflects how crucial supply management has become in modern drug research. Every clinical trial depends on a complex web of logistics everything from investigational drugs and testing kits to packaging, temperature control, and delivery. These supplies must be handled carefully to meet the demanding standards set by regulators and to protect both the safety of patients and the validity of the study. As trials expand across borders and involve larger patient pools, reliable supply systems have become essential to keep projects on track.Download Sample Report Here: https://www.meticulousresearch.com/request-sample-report/cp_id=5072
Growing Research Activity Driving the Market
The steady increase in clinical trials worldwide is the strongest engine of market growth. Pharmaceutical companies and biotech firms continue to push for innovation, investing heavily in research to bring new therapies to market. Governments are also supporting these efforts with major funding for biotechnology and advanced drug manufacturing. In 2023 alone, more than 477,000 registered clinical trials were active, compared to about 399,000 in 2021. Each of these trials depends on the timely movement of sensitive materials, some of which need round-the-clock temperature monitoring. Managing that process efficiently has become a science in itself, and demand for professional trial supply services has never been higher. The growing use of biopharmaceuticals and generic drugs adds another layer of opportunity. Biopharmaceuticals have changed how diseases are treated they're precise, effective, and often safer than older drug types. Generics, meanwhile, help keep healthcare affordable. Both rely on standardized, well-managed supplies to ensure consistent quality from development through approval.
Rising Complexity Across Trial Phases
Not all clinical trials are the same. Phase III, for example, is especially complex because it often involves thousands of participants across multiple countries. Managing supplies for a project of that scale while keeping every shipment compliant with local and international rules is no small task. It's one reason the demand for supply management services is strongest at this stage. Logistics and distribution make up the largest share of the industry. The need to transport drugs, biological samples, and other trial materials safely across borders has forced the sector to innovate. Companies are now using smarter tracking systems and digital tools that allow researchers to monitor shipments in real time, helping reduce delays and protect sensitive products from damage.
Browse in Depth: https://www.meticulousresearch.com/product/clinical-trial-supplies-market-5072
Market Segmentation
When the market is broken down by phase, Phase III trials clearly stand out because of their scale and cost. Phases I and II, which focus on early safety and efficacy testing, remain smaller but vital parts of the overall system.
By service, logistics and distribution lead the way, followed by packaging, labeling, manufacturing, and documentation services. The reason is simple transportation remains the toughest and most critical challenge for global studies.
Looking at product type, biologics are seeing the fastest rise in demand. However, small-molecule drugs and medical devices still account for a significant share of ongoing trials. The combination of traditional and next-generation products keeps the supply industry diverse and competitive.
In therapeutic areas, oncology dominates. The number of cancer drugs in development continues to climb, and this constant stream of research has turned oncology into the largest revenue segment for clinical trial supplies. Other important fields include cardiology, infectious diseases, neurology, metabolic disorders, and respiratory illnesses.
By end user, pharmaceutical and biotechnology firms represent the bulk of demand. Their extensive R&D investments and the pressure to bring new drugs to market quickly make them the biggest consumers of supply chain services. Medical device developers also depend heavily on these services to keep studies compliant and on schedule.
Regional Perspective
North America continues to lead the global market thanks to its advanced healthcare infrastructure, strong R&D funding, and a dense network of clinical research centers. High levels of healthcare spending and supportive regulations keep the region ahead of others. Europe follows closely, benefiting from its scientific expertise and long-standing culture of clinical collaboration. Meanwhile, Asia-Pacific is rapidly catching up. Countries like India, China, and Japan are hosting more clinical studies than ever, largely due to lower costs and growing medical capabilities. Emerging regions in Latin America, the Middle East, and Africa are also stepping into the spotlight as new destinations for global research, creating fresh demand for reliable trial supply systems.
Buy the Complete Report with an Impressive Discount: https://www.meticulousresearch.com/view-pricing/373
Conclusion
The clinical trial supplies market is becoming one of the most essential pieces of the global pharmaceutical ecosystem. As the number of studies increases and research grows more complex, the demand for efficient, transparent, and compliant supply chains will only intensify. Challenges remain drug development is expensive, regulations differ across regions, and trial failures can disrupt planning but opportunities are just as strong. The rise of biologics, the growth of generic drugs, and expanding R&D in emerging markets will keep the sector moving forward. In the coming years, innovation in logistics and cold-chain management will shape how the world conducts clinical trials. Those who can offer precision, reliability, and global reach will be at the heart of the next decade of medical progress.
Key questions answered in the report:
What compound annual growth rate (CAGR) is the market expected to achieve between 2024 and 2031?
What challenges does the market face despite its steady growth?
How does the increasing number of clinical trials worldwide contribute to market expansion?
What role do government funding and support play in driving research activity?
Which clinical trial phase accounts for the largest share of the market and why?
What distinguishes Phase III trials from earlier phases in terms of supply chain complexity?
Which region currently leads the global clinical trial supplies market, and what factors contribute to its dominance?
How is Europe positioned in comparison to North America?
Which product type is witnessing the fastest growth, and what drives this trend?
Why do small-molecule drugs and medical devices still hold a significant share of the market?
Related Reports:
Clinical Trial Management System (CTMS) Market: https://www.meticulousresearch.com/product/clinical-trial-management-system-market-5456
Clinical Trials Market: https://www.meticulousresearch.com/product/clinical-trials-market-5934
About Us: We are a trusted research partner for leading businesses worldwide, empowering Fortune 500 organizations and emerging enterprises with actionable market intelligence tailored to drive revenue transformation and strategic growth. Our insights reveal forward-looking revenue opportunities, providing our clients with a competitive edge through a diverse suite of research solutions-syndicated reports, custom research, and direct analyst engagement. Each year, we conduct over 300 syndicated studies and manage 60+ consulting engagements across eight key industry sectors and 20+ geographic markets. With a focus on solving the complex challenges facing global business leaders, our research enables informed decision-making that propels sustainable growth and operational excellence. We are dedicated to delivering high-impact solutions that transform business performance and fuel innovation in the competitive global marketplace.
Contact Us: Meticulous Market Research Pvt. Ltd. 1267 Willis St, Ste 200 Redding, California, 96001, U.S.
Email- sales@meticulousresearch.com
USA: +1-646-781-8004
Europe: +44-203-868-8738
APAC: +91 744-7780008
Visit Our Website: https://www.meticulousresearch.com/
For Latest Update Follow Us: LinkedIn- https://www.linkedin.com/company/meticulous-research
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Comprehensive Study on the Clinical Trial Supplies Market: Growth Drivers and Regional Dynamics | Meticulous Research® here
News-ID: 4269497 • Views: …
More Releases from Meticulous Research®
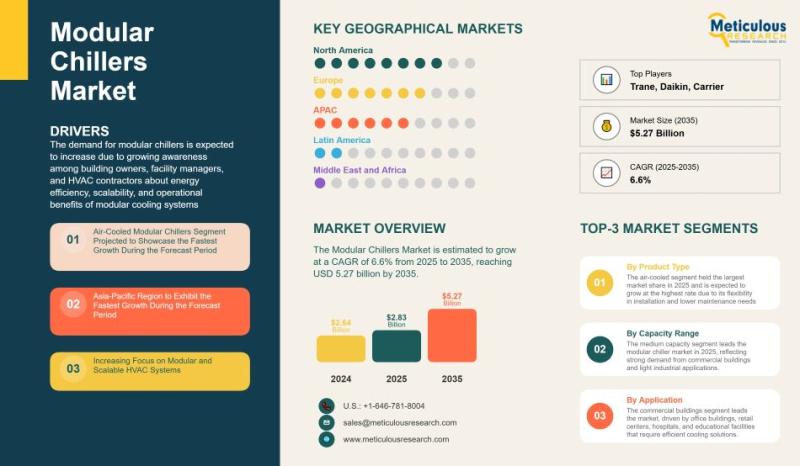
Global Modular Chillers Market: Trends, Technologies, and Growth Forecast 2025-2 …
The market for modular chillers has been growing steadily. In 2024, it was valued at roughly USD 2.64 billion, and it's expected to reach about USD 5.27 billion by 2035. This represents a growth rate of around 6.6% per year. The increase is mostly because building owners, facility managers, and HVAC professionals are starting to recognize the practical benefits of modular chillers. These systems are flexible, scalable, and can save…
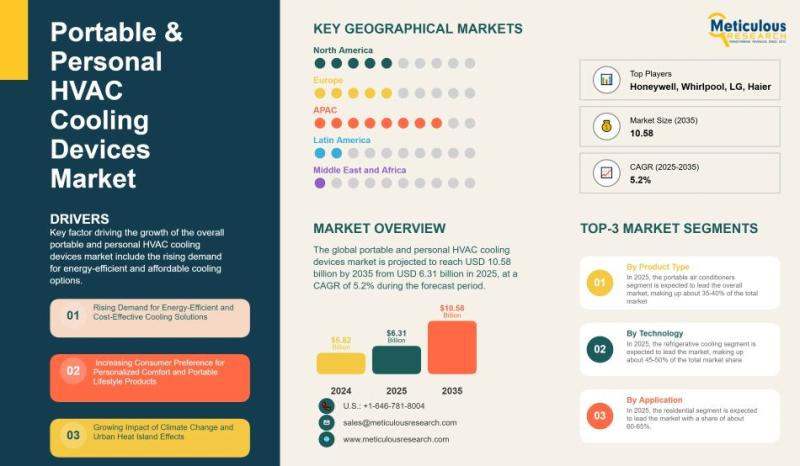
Global Portable and Personal HVAC Cooling Devices Market Outlook 2025-2035: Tren …
The global portable and personal HVAC cooling devices market is projected to grow from USD 6.31 billion in 2025 to USD 10.58 billion by 2035, at a CAGR of 5.2%. Valued at USD 5.82 billion in 2024, the market is expanding due to the rising preference for flexible, energy-efficient, and cost-effective cooling solutions. Consumers are increasingly prioritizing comfort, mobility, and lower energy costs, making portable cooling technologies an attractive alternative…
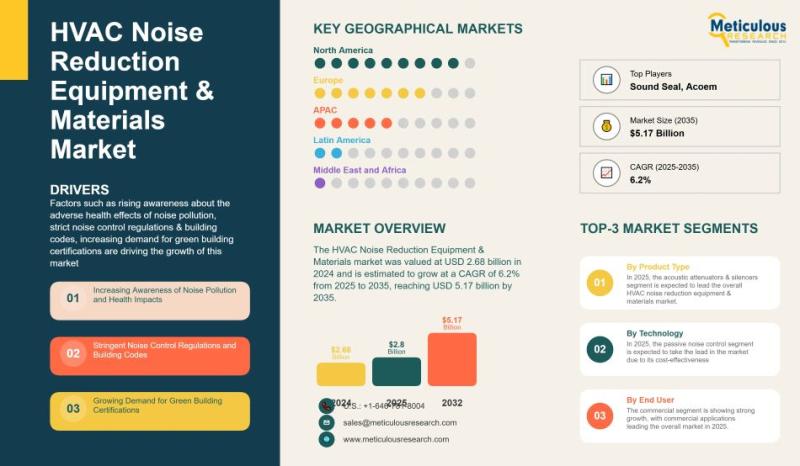
Global HVAC Noise Reduction Equipment & Materials Market Outlook 2025-2035: Tren …
The global HVAC Noise Reduction Equipment & Materials market has witnessed significant growth in recent years, reflecting the rising demand for quieter and more comfortable indoor environments. In 2024, the market was valued at approximately USD 2.68 billion and is projected to grow at a compound annual growth rate (CAGR) of 6.2% from 2025 to 2035, reaching an estimated USD 5.17 billion by 2035. This growth is driven by multiple…
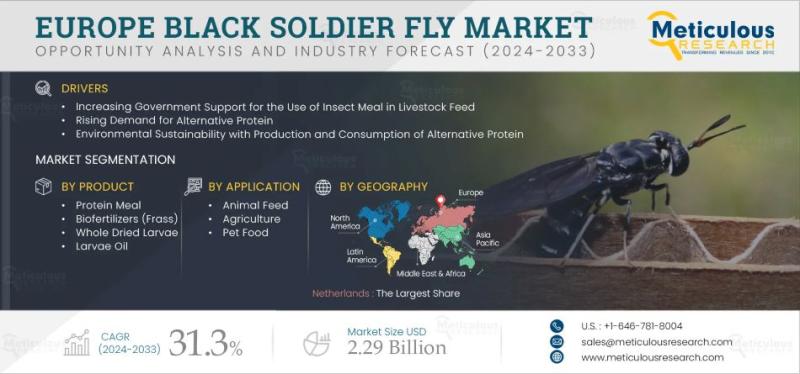
Europe Black Soldier Fly Market Outlook: Growth, Opportunities, and Trends 2025- …
The Europe Black Soldier Fly (BSF) market is projected to experience remarkable growth over the forecast period, driven by multiple factors that are reshaping the landscape of alternative protein production and sustainable feed solutions. Between 2025 and 2033, the market is expected to register a compound annual growth rate (CAGR) of 31.3% in terms of revenue, reaching an estimated value of $2.29 billion by 2033. From a volumetric perspective, the…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…