Press release
Regulatory Affairs Outsourcing Market Size Projected at USD 22.3 Billion by 2035, Fueled by Expanding Pharmaceutical and Biotechnology Pipelines
Market Size -The global Regulatory Affairs Outsourcing Market was valued at US$ 7.4 billion in 2024 and is projected to grow at a CAGR of 10.6% from 2025 to 2035, reaching more than US$ 22.3 billion by the end of 2035. The market's expansion is fueled by the increasing complexity of global regulatory frameworks, rising R&D investments by pharmaceutical and medical device companies, and growing demand for cost-effective solutions to accelerate product approvals and ensure compliance across diverse geographies.
👉 Do not miss the latest market intelligence. Get your sample report copy today@ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=3528
Market Overview -
The Regulatory Affairs Outsourcing Market involves third-party service providers assisting life science companies-such as pharmaceuticals, biotechnology firms, and medical device manufacturers-in navigating complex regulatory requirements. These services encompass product registration, clinical trial applications, labeling compliance, post-marketing surveillance, and regulatory strategy development.
Globalization of clinical trials, evolving regulatory policies, and the surge in new product development have compelled companies to partner with specialized outsourcing firms. Outsourcing enables faster submissions, ensures adherence to regional regulations (such as FDA, EMA, and CDSCO), and reduces overall operational costs.
Market Description -
Regulatory affairs outsourcing provides end-to-end support across a product's lifecycle-from development to commercialization. It allows companies to leverage the expertise of regulatory professionals to streamline documentation, compliance audits, and risk assessments.
With rapid advancements in biopharmaceuticals, personalized medicine, and medical devices, regulatory requirements have become increasingly stringent and region-specific. As a result, outsourcing has become a strategic approach to maintaining agility and global reach while minimizing compliance risks. The growing emphasis on digital transformation and automation in regulatory processes is further enhancing market growth, as companies adopt AI-driven documentation systems, regulatory information management (RIM) tools, and eCTD (electronic Common Technical Document) submissions.
Analysis of Key Players
Leading companies in the regulatory affairs outsourcing market are actively partnering with hospitals, specialty clinics, and research institutions to strengthen their market presence and achieve inorganic growth. Strategic collaborations and partnerships are enabling these organizations to enhance their service portfolios and expand geographically.
Prominent players operating in the market include
• Accell Clinical Research, LLC
• Genpact
• CRITERIUM, INC
• Promedica International
• WuXi AppTec
• Medpace
• Charles River Laboratories
• ICON plc
• Labcorp Drug Development
• Parexel International Corporation
• Freyr
• PHARMALEX GMBH
• Other Prominent Players.
Each of these companies has been comprehensively profiled in the Regulatory Affairs Outsourcing Market Research Report based on parameters such as company overview, financial performance, business strategies, product portfolio, business segments, and recent developments.
Key Developments
• August 2024: LEAP Consulting Group, a boutique digital consultancy serving the clinical laboratory industry, announced new services aimed at assisting CLIA-certified laboratories with planning and remediation processes. These services help laboratories extend their existing CLIA, CAP, and NYSDOH CLEP certifications to comply with the expanded requirements outlined in the U.S. FDA's Laboratory Developed Tests (LDT) Final Rule, issued in May 2024.
• October 2024: ProductLife Group (PLG), a global specialist in regulatory, scientific, compliance, and digital transformation consulting for the life sciences industry, announced the acquisition of Callisto, a UK-based consultancy firm. Callisto specializes in Regulatory Affairs (RA), Pharmacovigilance (PV), and GMDP services across multiple regulated sectors, including human and veterinary medicines, medical devices, and borderline products. This acquisition enhances PLG's service capabilities and expands its footprint in the European regulatory landscape.
👉 Discuss Implications for Your Industry Request Sample Research PDF@ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=3528
Key Player Strategies -
• Strategic Collaborations: Partnering with pharmaceutical and biotech companies for multi-regional regulatory support.
• Digital Transformation: Integrating AI and cloud-based RIM platforms to automate submission and compliance processes.
• Geographic Expansion: Establishing regulatory offices in emerging markets like Asia-Pacific and Latin America to support localized compliance.
• Comprehensive Service Offerings: Providing end-to-end solutions from clinical trial applications to post-marketing regulatory surveillance.
• Regulatory Intelligence: Using data analytics to forecast regulatory trends and proactively manage compliance strategies.
Challenges -
• Evolving Global Regulations: Frequent policy updates by regulatory authorities create compliance uncertainties.
• Data Security Concerns: Handling sensitive clinical and patient data poses privacy and cybersecurity challenges.
• Integration Barriers: Coordinating between in-house teams and outsourced partners can lead to communication and process gaps.
• Talent Shortage: Limited availability of skilled regulatory professionals impacts project timelines.
Opportunities -
• Growing Biopharmaceutical Pipeline: Increased drug approvals and biologics development boost regulatory documentation demand.
• Emerging Markets Expansion: Rising healthcare investments in Asia-Pacific and Latin America open new outsourcing avenues.
• Digital Regulatory Platforms: Adoption of AI, cloud, and RIM systems enhances process efficiency and transparency.
• Post-Market Compliance Services: Growing focus on pharmacovigilance and lifecycle management creates long-term service opportunities.
• SME Demand Surge: Small and mid-sized pharma firms increasingly outsource regulatory affairs to reduce cost burdens.
Market Segmentations -
By Service Type:
• Regulatory Submissions
• Regulatory Writing & Publishing
• Clinical Trial Applications & Approvals
• Product Registration & Market Authorization
• Regulatory Consulting
• Legal Representation
• Post-Marketing Surveillance
By Industry:
• Pharmaceuticals
• Biotechnology
• Medical Devices
• Food & Nutraceuticals
• Cosmetics
By Stage:
• Preclinical
• Clinical
• Post-Approval
By End User:
• Large Enterprises
• Small & Medium Enterprises (SMEs)
By Region:
• North America: Leading market with strong presence of global CROs and high regulatory complexity.
• Europe: Significant share driven by EMA harmonization initiatives and growth in biosimilars.
• Asia-Pacific: Fastest-growing region with expanding pharmaceutical manufacturing and clinical research hubs.
• Latin America & Middle East & Africa: Emerging regions with increasing demand for product registration and compliance services.
👉 To buy this comprehensive market research report, click here to inquire@ https://www.transparencymarketresearch.com/checkout.php?rep_id=3528<ype=S
Why Buy This Report?
• Comprehensive Market Insights: Understand the dynamics shaping the global regulatory affairs outsourcing landscape.
• Detailed Segment Analysis: Explore service-wise and regional growth patterns with actionable intelligence.
• Competitive Benchmarking: Learn from the strategies of key global players and new entrants.
• Accurate Forecasts: Access market projections to support strategic decision-making and expansion planning.
• Technology Integration Insights: Identify opportunities for digital transformation in regulatory workflows.
• Strategic Recommendations: Gain expert guidance to address compliance challenges and capitalize on outsourcing growth trends.
Conclusion -
The Regulatory Affairs Outsourcing Market is set for strong growth, driven by increasing regulatory complexity, globalization of drug development, and the expanding pipeline of innovative therapies. Outsourcing enables life science companies to remain compliant, agile, and cost-efficient while focusing on core R&D activities. As the industry transitions toward digital-first compliance systems and AI-enabled documentation, strategic partnerships with experienced regulatory service providers will be critical to sustaining long-term success.
More Trending Research Reports-
• Positron Emission Tomography [PET] Scanners Market - https://www.transparencymarketresearch.com/positron-emission-tomography-pet-scanners-market.html
• Blood Testing Market - https://www.transparencymarketresearch.com/blood-testing-market.html
About Us Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. The firm scrutinizes factors shaping the dynamics of demand in various markets. The insights and perspectives on the markets evaluate opportunities in various segments. The opportunities in the segments based on source, application, demographics, sales channel, and end-use are analysed, which will determine growth in the markets over the next decade.
Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision-makers, made possible by experienced teams of Analysts, Researchers, and Consultants. The proprietary data sources and various tools & techniques we use always reflect the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in all of its business reports.
Contact Us
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Blog: https://tmrblog.com
Email: sales@transparencymarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Regulatory Affairs Outsourcing Market Size Projected at USD 22.3 Billion by 2035, Fueled by Expanding Pharmaceutical and Biotechnology Pipelines here
News-ID: 4269160 • Views: …
More Releases from Transparency Market Research Pvt Ltd
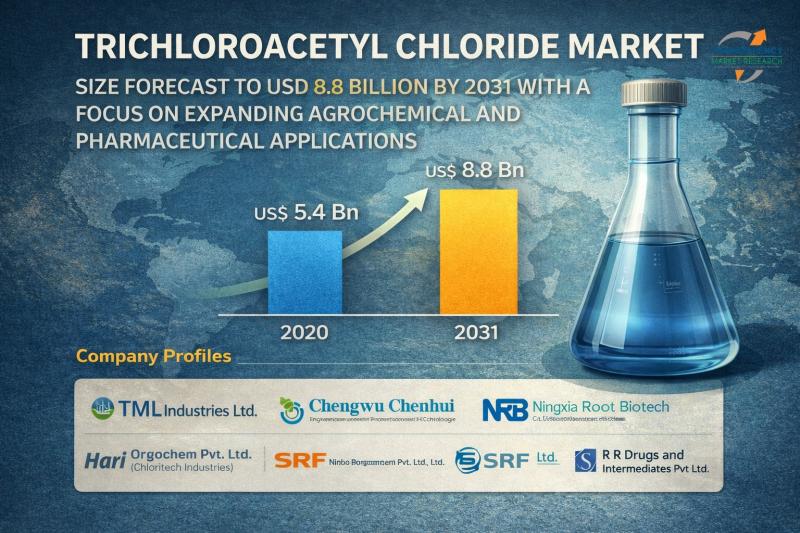
Trichloroacetyl Chloride Market Size Forecast to USD 8.8 Billion by 2031 with a …
Trichloroacetyl Chloride Market Market Size to 2031
The global trichloroacetyl chloride market was valued at over US$ 5.4 billion in 2020. It is estimated to expand at a CAGR of 4.6% from 2021 to 2031, and is expected to surpass US$ 8.8 billion by the end of 2031. Sustained growth is driven by increasing industrial chemical demand, rising pharmaceutical and agrochemical production, and expanding applications in specialty chemical synthesis worldwide.
👉 Get…
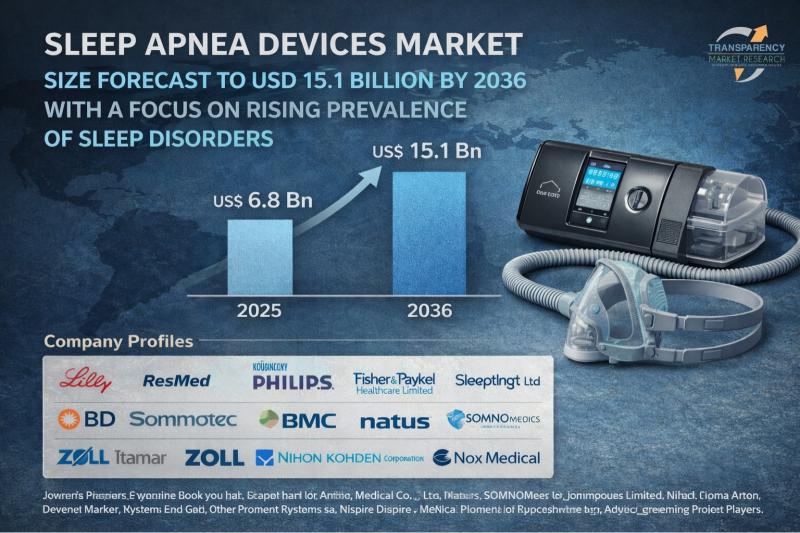
Sleep Apnea Devices Market Size Forecast to USD 15.1 Billion by 2036 with a Focu …
Sleep Apnea Devices Market Outlook 2036
The global sleep apnea devices market was valued at US$ 6.8 Bn in 2025 and is projected to reach US$ 15.1 Bn by 2036, expanding at a steady CAGR of 7.5% from 2026 to 2036. Market growth is primarily driven by the rising prevalence of obstructive sleep apnea (OSA), increasing awareness about sleep disorders, and technological advancements in positive airway pressure (PAP) devices and wearable…
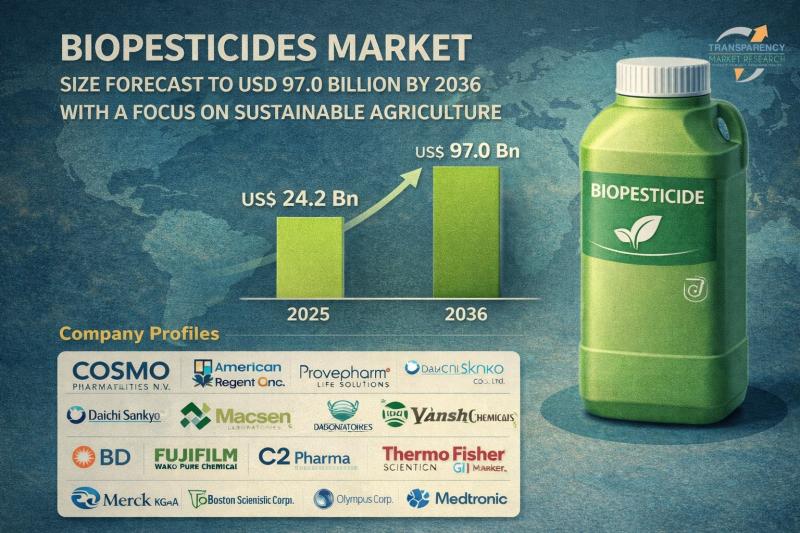
Biopesticides Market Size Forecast to USD 97.0 Billion by 2036 with a Focus on S …
Biopesticides Market Outlook 2036
The global biopesticides market was valued at US$ 24.2 Bn in 2025 and is projected to reach US$ 97.0 Bn by 2036, expanding at a robust CAGR of 13.5% from 2026 to 2036. The market growth is primarily driven by the increasing shift toward sustainable agriculture, rising demand for organic food products, and stringent regulations on synthetic chemical pesticides worldwide.
👉 Do not miss the latest market intelligence.…

Mono Cartons Market Size Forecast to USD 1.0 Billion by 2031 with Rising Demand …
Mono Cartons Market Outlook 2031
The global mono cartons market was valued at US$ 723.1 million in 2022. The market is projected to expand at a CAGR of 4.4% from 2023 to 2031, reaching US$ 1.0 billion by the end of 2031. Growth is supported by rising demand for sustainable packaging solutions, expansion of the FMCG and pharmaceutical sectors, and increasing preference for attractive, lightweight, and recyclable packaging formats.
👉 Get your…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…
