Press release
United States Decentralized Clinical Trials Market valuation $29.90 billion by 2033 - Exclusive Report by DatamIntelligence
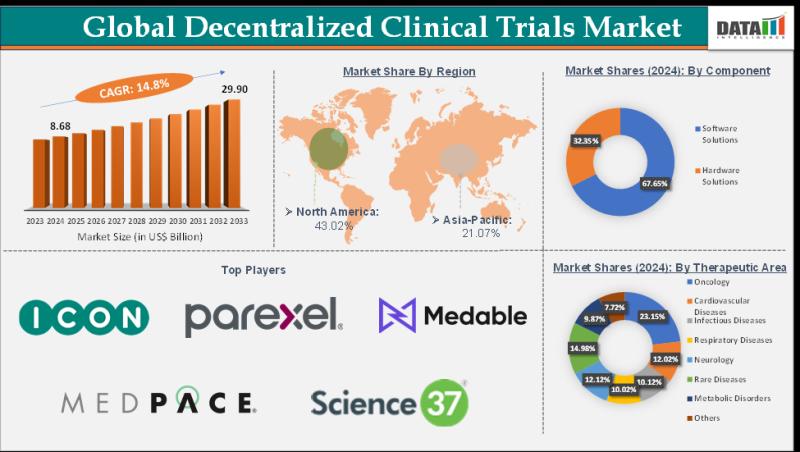
"Decentralized Clinical Trials Market Size reached US$ 8.68 Billion in 2024 and is expected to reach US$ 29.90 Billion by 2033, growing at a CAGR of 14.8% during the forecast period 2025-2033." As per DataM intelligence research reportDownload your exclusive sample report today: (corporate email gets priority access): https://www.datamintelligence.com/download-sample/decentralized-clinical-trials-market?sp
United States: Recent Industry Developments
✅ In October 2025, Pfizer expanded its decentralized clinical trial platform for oncology studies, integrating remote patient monitoring and telehealth visits. The approach reduces travel burden and increases patient retention. It supports faster, more inclusive clinical trial enrollment.
✅ In September 2025, Medable launched new tools for DCTs enabling eConsent, remote data collection, and wearable device integration. The platform improves data quality and trial efficiency. It empowers patients to participate from home and community sites.
✅ In August 2025, Johnson & Johnson completed a multi-site DCT pilot for rare disease therapeutics, leveraging mobile health apps and remote lab sampling. The trial improved participant adherence and real-time monitoring. It demonstrates the potential of DCTs in complex therapeutic areas.
✅ In July 2025, ICON plc partnered with telehealth providers to enhance decentralized clinical trial capabilities for cardiovascular studies. Remote monitoring and virtual visits reduce site dependency. The initiative supports regulatory compliance and patient-centric trial design.
Japan: Recent Industry Developments
✅ In October 2025, Takeda Pharmaceutical launched DCT initiatives for oncology trials in Japan, integrating wearable biosensors and remote patient reporting. The system reduces hospital visits while maintaining data integrity. It aligns with Japan's push for patient-centric clinical research.
✅ In September 2025, Astellas Pharma adopted decentralized trial protocols for early-phase studies, incorporating telemedicine and home-based sample collection. The approach improves patient convenience and enrollment rates. It enhances trial flexibility and operational efficiency.
✅ In August 2025, CMIC Holdings implemented a hybrid DCT platform combining site visits with remote monitoring for metabolic disorder trials. The system enables continuous data capture and patient engagement. It supports more efficient and inclusive clinical research.
✅ In July 2025, PPD Japan partnered with digital health providers to enable remote monitoring and eConsent in decentralized clinical trials. The initiative increases participation among patients in rural areas. It enhances trial accessibility and patient-centered design.
Decentralized Clinical Trials Market: Drivers
The decentralized clinical trials (DCT) market is experiencing rapid growth as pharmaceutical companies, contract research organizations (CROs), and healthcare providers increasingly adopt patient-centric, remote, and technology-driven trial models. DCTs leverage digital tools, telemedicine, wearable devices, and electronic patient-reported outcomes to conduct clinical research outside traditional sites, improving patient access, engagement, and retention. Rising demand for faster drug development, cost-effective trial management, and real-time data collection is driving adoption. Additionally, the COVID-19 pandemic accelerated the shift toward virtual and hybrid clinical trials, highlighting the benefits of decentralized approaches. Regulatory support, technological advancements, and growing patient awareness of clinical trial participation are further supporting market expansion.
Technological innovations in remote monitoring, mobile health apps, electronic data capture, and AI-driven analytics are enhancing the efficiency, accuracy, and scalability of decentralized clinical trials. Collaborations between technology providers, sponsors, and healthcare institutions are fostering the development of integrated digital trial platforms. Increasing focus on patient convenience, diversity in clinical trial populations, and adherence to real-world data collection is driving adoption. Expansion of telehealth infrastructure and cloud-based systems is improving accessibility and operational efficiency. With continuous innovation and rising emphasis on patient-centric, agile clinical research, the decentralized clinical trials market is poised for sustained global growth.
Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/decentralized-clinical-trials-market?sp
Decentralized Clinical Trials Market: Major Players
ICON plc, Parexel International Corporation, Laboratory Corporation of America Holdings, Medpace, Inc., Science 37, THREAD, Inc., Curebase, Castor, IQVIA Inc., and Medable Inc., among others.
Segment Covered in the Decentralized Clinical Trials Market:
By Study Type:
Interventional trials dominate, leveraging remote patient monitoring and telemedicine for efficient data collection. Observational trials grow due to lower cost and broader patient participation. Expanded access trials gain traction in rare and emergency-use cases.
By Component:
Software solutions lead the segment with digital platforms enabling virtual recruitment, eConsent, and remote monitoring. Hardware solutions, including wearable devices and sensors, support real-time patient data tracking. Integration between both components enhances trial efficiency.
By Therapeutic Area:
Oncology holds the largest share driven by high patient diversity and remote data needs. Cardiovascular and infectious disease trials rapidly expand with rising chronic and pandemic-related research. Rare and metabolic disorders benefit from improved patient accessibility through DCT models.
By End-User:
Pharmaceutical and biotechnology companies dominate as they adopt virtual trials for faster drug development. Contract research organizations (CROs) increasingly deploy hybrid models to meet sponsor demands. Academic institutes embrace decentralized tools for broader research participation.
Regional Analysis
North America (≈42% share):
Leads the market owing to robust regulatory support and advanced telehealth infrastructure. The U.S. spearheads innovation through FDA guidance on remote trials. Strong collaboration between pharma companies and digital health firms fuels growth.
Europe (≈28% share):
Second-largest region supported by EMA's decentralized trial frameworks. The U.K., Germany, and France are early adopters of hybrid clinical models. Emphasis on data privacy and interoperability strengthens regional implementation.
Asia Pacific (≈18% share):
Fastest-growing region driven by large patient pools and mobile technology penetration. China, Japan, and India promote remote monitoring for cost-efficient clinical research. Investments in digital health startups accelerate market expansion.
South America (≈7% share):
Moderate growth led by Brazil and Argentina, focusing on improving clinical trial access. Adoption of remote data collection tools enhances efficiency. Regulatory harmonization with global standards supports trial decentralization.
Middle East & Africa (≈5% share):
Emerging market witnessing gradual adoption of decentralized models. Growing healthcare digitalization and remote trial platforms expand reach to diverse patient populations. Local government initiatives improve research capabilities.
Purchase this report before year-end and unlock an exclusive 30% discount:
https://www.datamintelligence.com/buy-now-page?report=decentralized-clinical-trials-market
(Purchase 2 or more Repots and get 50% Discount)
Request for 2 Days FREE Trial Access:
https://www.datamintelligence.com/reports-subscription?sp
✅ Competitive Landscape
✅ Technology Roadmap Analysis
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Consumer Behavior & Demand Analysis
✅ Import-Export Data Monitoring
✅ Live Market & Pricing Trends
Have a look at our Subscription Dashboard:
https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release United States Decentralized Clinical Trials Market valuation $29.90 billion by 2033 - Exclusive Report by DatamIntelligence here
News-ID: 4264066 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
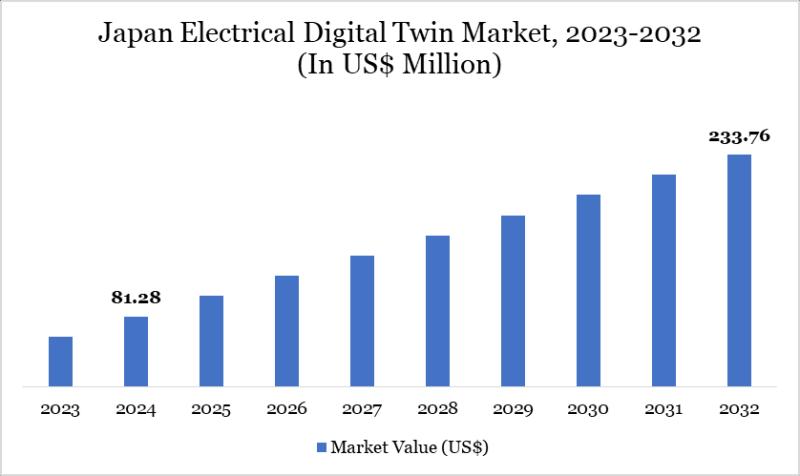
Japan Electrical Digital Twin Market 2026 | Growth Drivers, Key Players & Invest …
Market Size and Growth
Japan Electrical Digital Twin Market Size reached US$ 81.28 million in 2024 and is expected to reach US$ 233.76 million by 2032, growing with a CAGR of 13.6% during the forecast period 2025-2032.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/japan-electrical-digital-twin-market?sb
Key Development:
Japan: Recent Electrical Digital Twin Developments
✅ In January 2026, The University of Tokyo and Fujitsu launched Japan's first…
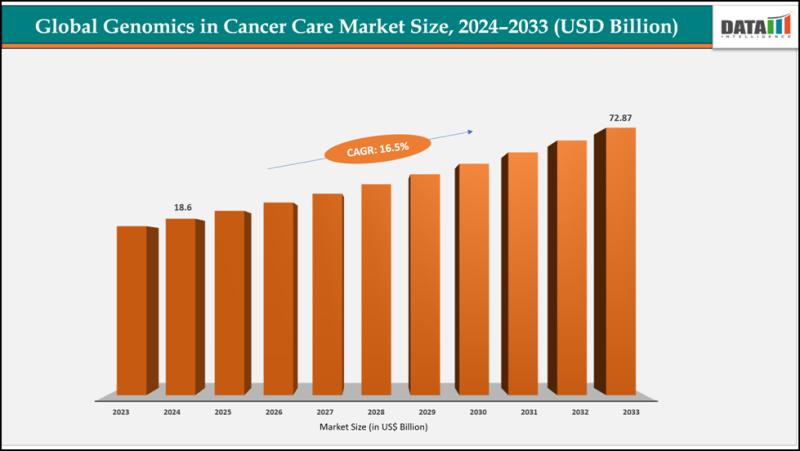
United States Genomics in Cancer Care Market 2026 | Growth Drivers, Key Players …
Market Size and Growth
The global Genomics in cancer care market size reached US$ 16.14billion with rise of US$18.6billion in 2024 is expected to reach US$ 72.87billion by 2033, growing at a CAGR of 16.8%during the forecast period 2025-2033.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/genomics-in-cancer-care-market?sb
Key Development:
United States: Recent Industry Developments
✅ In January 2026, 10x Genomics and the Cancer Research Institute launched a…

United States Paint Protection Films Market 2026 | Growth Drivers, Trends & Mark …
Market Size and Growth
The Global Paint Protection Films Market is estimated to reach at a high CAGR during the forecast period (2024-2031).
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/paint-protection-films-market?sb
Key Development:
United States: Recent Industry Developments
✅ In January 2026, XPEL highlighted a national dealership survey showing paint protection film can increase vehicle resale value by up to 15%, reinforcing the value proposition of…

Biochemistry Analysers Market is Expected to Reach US$ 8,275.48 Million by 2033 …
Market Overview:
The global biochemistry analysers market reached US$ 4,658.67 Million in 2023, rising to US$ 4,881.15 Million in 2024, and is projected to reach US$ 8,275.48 Million by 2033, growing at a CAGR of 6.1% during the forecast period 2025-2033. The market growth is fueled by technological advancements, rising disease prevalence, and increasing demand for automated diagnostic tools. Biochemistry analysers play a vital role in clinical laboratories by analyzing blood…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…