Press release
Emerging Trends to Reshape the Global Medical Device Testing, Inspection, And Certification Market: Focus On Advanced Third-Party Verification And Validation Services To Build Trust And Ensure Compliance as a Key Influencer
Use code ONLINE20 to get 20% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.Medical Device Testing, Inspection, And Certification Market Size Valuation Forecast: What Will the Market Be Worth by 2025?
The segment dedicated to testing, examining, and certifying medical apparatus has experienced robust expansion over the preceding years, projected to ascend from a valuation of $7.97 billion in 2024 to $8.64 billion the following year, reflecting an 8.4% compound annual growth rate. This upward trajectory during the review period stems from several key factors, including a greater incorporation of biocompatibility assessments for emerging materials, the wider implementation of inspection systems that leverage automation and optics, escalating needs for validation testing related to software and cybersecurity, heightened focus on confirming sterilization protocols and contamination management, and a growing necessity for continuous quality oversight integrated directly into the manufacturing processes for these devices.
Medical Device Testing, Inspection, And Certification Market Size Forecast: What's the Projected Valuation by 2029?
Projected to achieve substantial expansion across coming years, the market encompassing testing, inspection, and certification services for medical devices is anticipated to reach a valuation of $11.81 billion by 2029, demonstrating an annual growth rate consistently calculated at 8.1%. This upward trajectory throughout the projection span is fueled by several key factors: stricter mandates regarding regulatory adherence for apparatuses, a growing consumer and industry need for medical items that are both safe and of superior quality, greater uptake of sophisticated inspection and testing methodologies, heightened emphasis placed upon mitigating risks and ensuring patient well-being, alongside escalating capital allocations toward innovation and development within the medical device sector. Key directional shifts anticipated over this period involve the progression of automated and robotics-driven inspection frameworks, the utilization of sophisticated artificial intelligence tools to forecast quality outcomes, novel developments in methods that allow testing without structural compromise, incorporating digital replicas for validating how devices function, and movement towards global convergence of requirements for official certification.
View the full report here:
https://www.thebusinessresearchcompany.com/report/medical-device-testing-inspection-and-certification-global-market-report
What Are the Drivers Transforming the Medical Device Testing, Inspection, And Certification Market?
Anticipated increases in spending on healthcare are projected to be the impetus behind the expansion of the sector encompassing testing, inspection, and certification services for medical devices. This spending constitutes the monetary investment made by governmental bodies, private organizations, or private citizens to deliver medical care, administer treatments, and engage in health-related functions. The upward trend in healthcare outlay is attributable to the augmenting incidence of long-term ailments that necessitate continuous and expensive care regimens. Such expenditures directly underpin the necessary quality assurance, regulatory adherence, and development funding vital for medical device evaluation and certification processes, ensuring technologies are both safe and efficacious. To illustrate this dynamic, the UK's Office for National Statistics reported in May 2024 that nominal total healthcare spending escalated by 5.6% between 2022 and 2023, representing a marked acceleration when contrasted with the modest 0.9% growth recorded throughout 2022. Consequently, the escalating financial commitment within the healthcare domain is propelling the advancement of the medical device testing, inspection, and certification market.
Get your free sample here:
https://www.thebusinessresearchcompany.com/sample.aspx?id=29120&type=smp
What Long-Term Trends Will Define the Future of the Medical Device Testing, Inspection, And Certification Market?
Key players within the medical device testing, inspection, and certification sector are prioritizing the creation of sophisticated offerings, like independent verification and validation, with the goal of bolstering confidence among clients, regulatory bodies, and interested parties. This concept of third-party verification and validation involves an impartial entity assessing and confirming adherence of a product, procedure, or system to established criteria or necessary specifications. To illustrate this commitment, in the month of October 2023, UL Solutions, which is an American firm providing TIC services, initiated medical device testing capabilities at its Rochester Hills, Michigan laboratory location. These newly available services in Michigan serve to bolster assistance for the medical device manufacturing growth occurring within the state through improvements in product safety, protection, ease of use, and standardized compatibility. The Rochester Hills site now facilitates thorough auditing, cybersecurity provisions, usability assessments, compliance instruction for producers, alongside the core third-party verification and validation processes. It boasts adaptable testing options, encompassing accelerated durability and environmental assessments, all customized to suit particular manufacturer needs. Furthermore, the site's design incorporates the maintenance of a controlled atmosphere with minimized volatile organic compounds (VOCs), thereby guaranteeing superior precision and dependability in testing outcomes.
Which Segments in the Medical Device Testing, Inspection, And Certification Market Offer the Most Profit Potential?
The medical device testing, inspection, and certificationmarket covered in this report is segmented -
1) By Device Type: Active Medical Devices, Non-active Medical Devices, In-vitro Diagnostic Devices, Combination Products
2) By Testing Type: Safety Testing, Performance Testing, Biocompatibility Testing, Efficacy Testing
3) By Inspection Type: Component Inspection, Final Product Inspection, Quality System Inspection, Compliance Inspection
4) By Certification Type: International Organization For Standardization (ISO) Certification, Conformity European (CE) Marking, Food And Drug Administration (FDA) Approval, Other Regulatory Certifications
5) By End-User: Medical Device Manufacturers, Regulatory Authorities, Healthcare Facilities, Independent Testing Laboratories
Subsegments:
1) By Active Medical Devices: Diagnostic Imaging Devices, Patient Monitoring Devices, Therapeutic Devices, Surgical Devices
2) By Non-active Medical Devices: Surgical Instruments, Wound Care Products, Medical Consumables, Hospital Furniture And Equipment
3) By In-vitro Diagnostic Devices: Reagents And Kits, Analytical Instruments, Molecular Diagnostics Devices, Point-Of-Care Testing Devices
4) By Combination Products: Drug-Device Combination Products, Biologic-Device Combination Products, Device-Device Combination Products
Tailor your insights and customize the full report here:
https://www.thebusinessresearchcompany.com/customise?id=29120&type=smp
Which Firms Dominate the Medical Device Testing, Inspection, And Certification Market by Market Share and Revenue in 2025?
Major companies operating in the medical device testing, inspection, and certification market are Société Générale de Surveillance S.A., Eurofins Scientific S.E., Bureau Veritas S.A., Intertek Group plc, Charles River Laboratories International Inc., TÜV SÜD Aktiengesellschaft, UL Solutions Inc., Element Materials Technology Limited, QIMA Limited, Cotecna Inspection SA, North American Science Associates LLC, TÜV Rheinland AG, Freyr Solutions Pvt. Ltd., Nelson Laboratories LLC, MED Institute Inc., Auriga Research Limited, DDL Inc., F2 Labs LLC, DeviceLab LLC, and EdgeOne Medical Inc.
Which Regions Offer the Highest Growth Potential in the Medical Device Testing, Inspection, And Certification Market?
North America was the largest region in the medical device testing, inspection, and certification market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the medical device testing, inspection, and certification market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Purchase the full report today:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=29120
This Report Supports:
1.Business Leaders & Investors - To identify growth opportunities, assess risks, and guide strategic decisions.
2.Manufacturers & Suppliers - To understand market trends, customer demand, and competitive positioning.
3.Policy Makers & Regulators - To track industry developments and align regulatory frameworks.
4.Consultants & Analysts - To support market entry, expansion strategies, and client advisory work.
Reach out to us:
The Business Research Company: https://www.thebusinessresearchcompany.com/,
Americas +1 310-496-7795,
Europe +44 7882 955267,
Asia & Others +44 7882 955267 & +91 8897263534,
Email us at info@tbrc.info.
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company,
Twitter: https://twitter.com/tbrc_info,
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Learn More About The Business Research Company
With over 17500+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead.Our flagship product, the Global Market Model (GMM), is a premier market intelligence platform delivering comprehensive and updated forecasts to support informed decision-making.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Emerging Trends to Reshape the Global Medical Device Testing, Inspection, And Certification Market: Focus On Advanced Third-Party Verification And Validation Services To Build Trust And Ensure Compliance as a Key Influencer here
News-ID: 4263002 • Views: …
More Releases from The Business Research Company
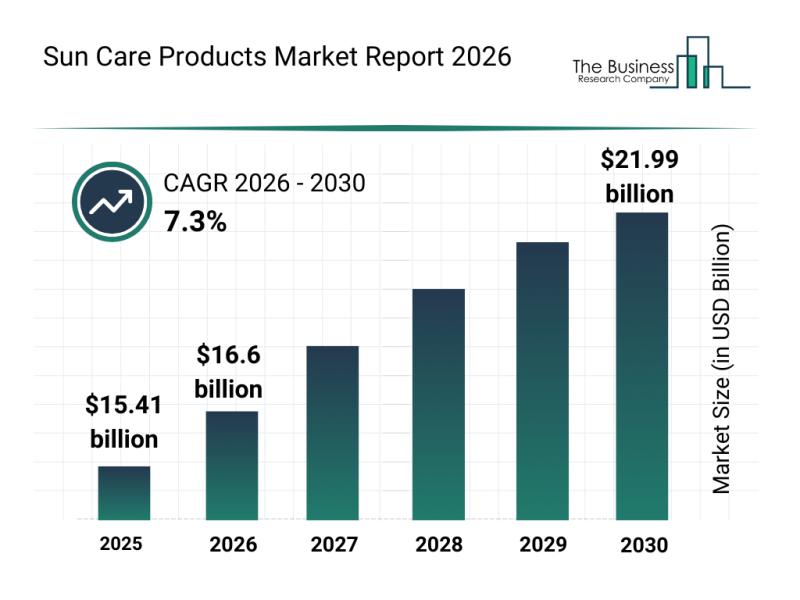
Leading Companies Fueling Growth and Innovation in the Sun Care Products Market
The sun care products market is on track for substantial expansion as consumer awareness about skin protection intensifies worldwide. With evolving preferences and technological advancements shaping product offerings, this sector is set to witness robust growth in the coming years. Let's explore the market's size projections, key players, emerging trends, and major segments driving its development through 2030.
Projected Size and Growth Trajectory of the Sun Care Products Market
The…
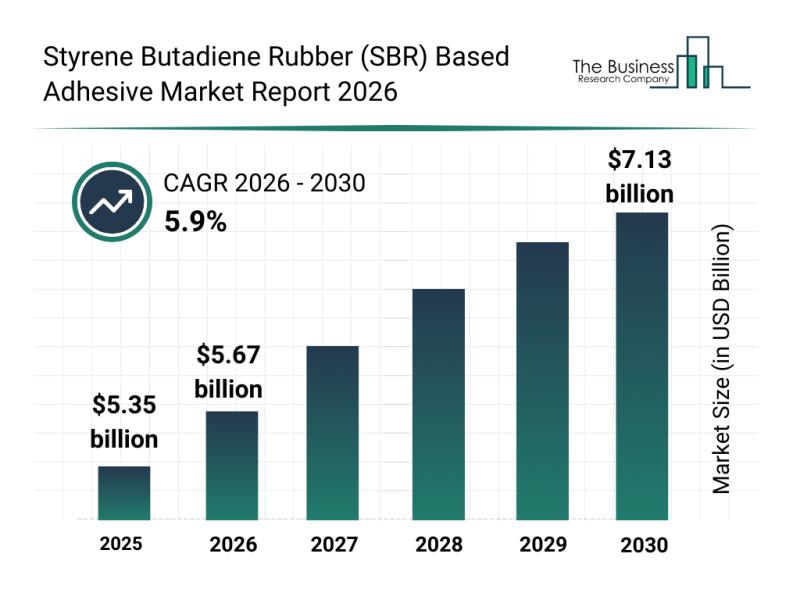
Future Perspectives: Key Trends Shaping the Styrene Butadiene Rubber (SBR) Based …
The styrene butadiene rubber (SBR) based adhesive market is on track for notable growth as we approach 2030. Driven by a variety of factors including expanding infrastructure projects and rising demand across multiple industries, this sector is poised for steady expansion. Let's explore the market's size projections, key players, emerging trends, and the main segments shaping its future.
Projected Growth and Market Size of Styrene Butadiene Rubber Based Adhesives
The…
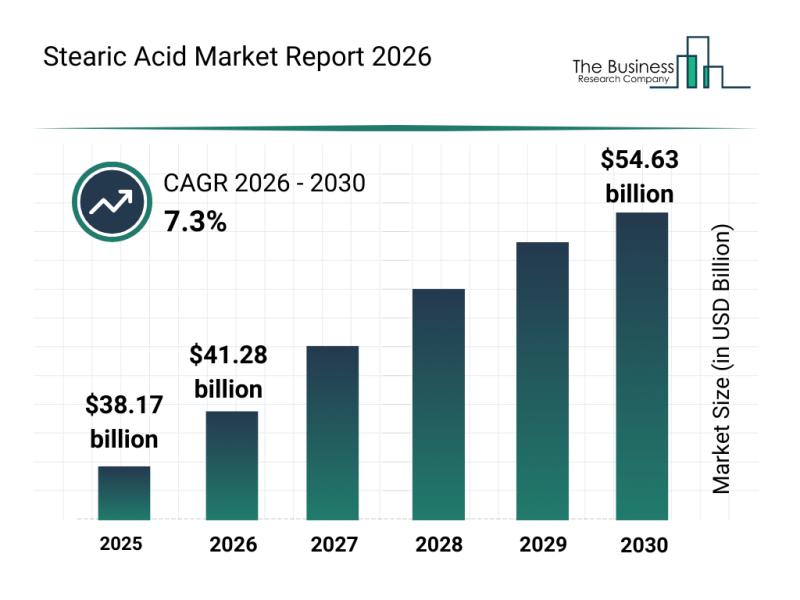
Emerging Sub-Segments Transforming the Stearic Acid Market Landscape
The stearic acid market is poised for significant expansion in the coming years, driven by evolving demand across various industries. This report explores the projected market size, leading companies, key trends, and segment analysis shaping the future of this vital chemical.
Stearic Acid Market Size and Growth Outlook
The stearic acid market is set to grow robustly, reaching a valuation of $54.63 billion by 2030. This represents a compound annual…
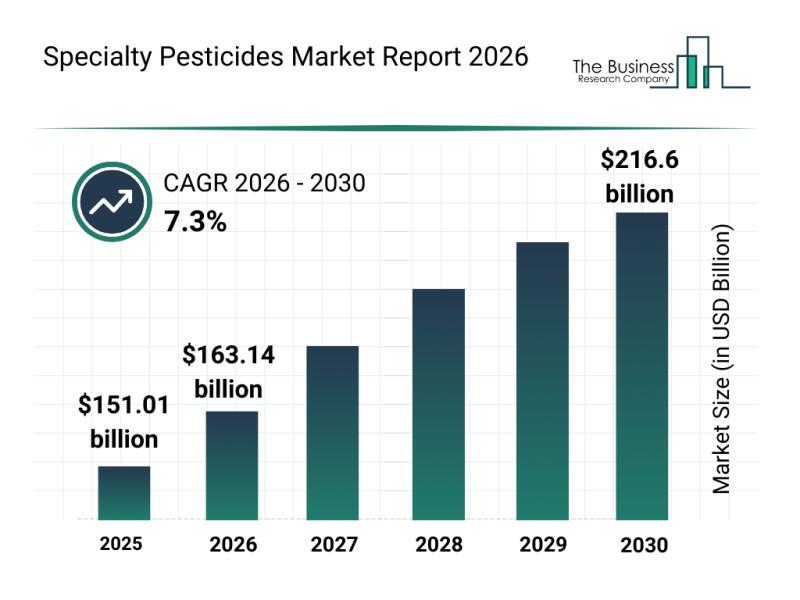
Market Trend Insights: The Impact of Recent Innovations on the Specialty Pestici …
The specialty pesticides sector is on the verge of significant expansion as global agricultural practices continue to evolve. Driven by increasing demand for crop protection and sustainable farming techniques, this market is set to experience robust growth in the coming years. Let's explore the market's anticipated value, leading companies, emerging trends, and detailed segmentation to gain a comprehensive understanding of this dynamic industry.
Projected Market Size and Growth Expectations for Specialty…
More Releases for Device
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Surge In Wireless Device Usage Boosts Wireless Audio Device Market Driving Marke …
Stay ahead with our updated market reports featuring the latest on tariffs, trade flows, and supply chain transformations.
How Large Will the Wireless Audio Device Market Size By 2025?
In recent years, there has been remarkable growth in the wireless audio device market size. The market, which is projected to expand from $41.85 billion in 2024 to $52.37 billion in 2025, boasts a compound annual growth rate (CAGR) of 25.1%. Factors contributing…
Anti-snoring Device Market - Quiet Nights, Restful Sleep: Anti-snoring Device In …
Newark, New Castle, USA: The "Anti-snoring Device Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Anti-snoring Device Market: https://www.growthplusreports.com/report/antisnoring-device-market/8931
This latest report researches the industry structure, sales, revenue,…
Global Watch Clock Measuring Device Market | Watch Clock Measuring Device Indust …
Watch, clock and measuring device market comprises of the sales of watch, clock, measuring device & related services to measure the time and physical quantity. Watch is portable timepiece, which is worn by people around the wrist, attached by a strap. Clock is a device used to measure and indicate time, using the pointers moving over a dial. Measuring device is an instrument used for measuring the various parameters in…
Peripheral Vascular Device Market Size, Peripheral Vascular Device Market Share, …
Global Peripheral Vascular Device Market Size is observed to gain traction owing to the factors such as increasing research and development for developing several new product, and rising funding by the private organizations.
Request for Sample of This Research Report @ https://bit.ly/2xjOKpC
Top Key Player:-
Abbott Laboratories
Braun Melsungen AG
Boston Scientific Corporation
R. Brad, Inc.
Cardinal Health, Inc.
Medtronic plc.
Cook Medical, Inc.
Teruma Corporation
Jude Medical, Inc.
The Spectranetics Corporation
Volcano Corporation
Peripheral vascular disorder (PVD) is a blood circulation disorder…
Medical Device Technologies Market - The Evolution of Medical Device Technologie …
The global medical device technologies market is anticipated to be boosted by various well-known players in the market. Some of these players that are dealing with the manufacturing of in vitro diagnostic devices hold a significant share in the global market. Whereas, the small market players are emerging from several developing nations, looking to set their foot in the market. Such measures are foreseen to change the market scenario in…
