Press release
Gene Therapy for Ultra-Rare Diseases Market Forecast 2035 | Key Driver, Restraint, and Growth Opportunity
Gene Therapy for Ultra-Rare Diseases Market Forecast 2035 | Key Driver, Restraint, and Growth Opportunity
Get the Detailed Industry Analysis (including the Table of Contents, List of Figures, and List of Tables) - from the Gene Therapy for Ultra-Rare Diseases Market Research Report: https://marketgenics.co/press-releases/gene-therapy-for-ultra-rare-diseases-market-76271
Global Gene Therapy for Ultra-Rare Diseases Market Forecast 2035:
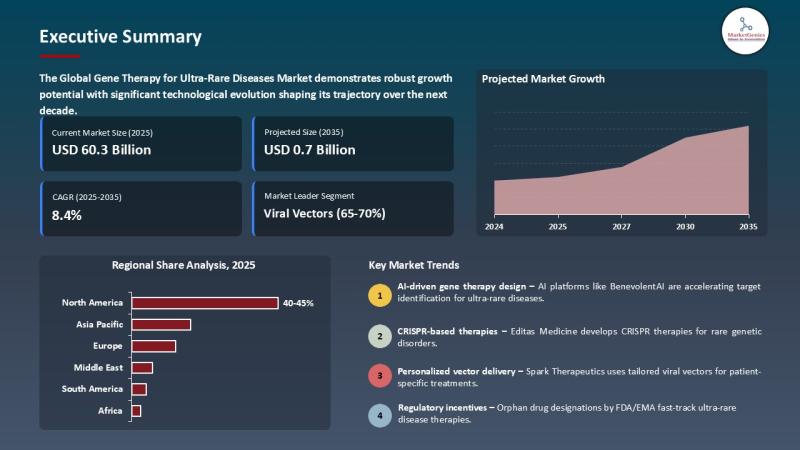
According to the report, the global gene therapy for ultra-rare diseases market is projected to expand from USD 0.3 billion in 2025 to USD 0.7 billion by 2035, registering a CAGR of 8.4%, the highest during the forecast period. The market of Gene Therapy of Ultra-Rare Diseases is growing at a rapid pace globally, driven by the improvement of viral and non-viral gene delivery approaches, the expansion of activities of CRISPR and other gene-editing technologies, and the increased focus on individualized medicine toward patients with ultra-rare genetic disorders. The increased occurrence of rare genetic diseases, in combination with more awareness in patients and a positive regulatory environment, is hastening the creation, clinical studies, and gains of new treatments.
North America is already the market leader because of its well-developed biotechnology base and the presence of excellent clinical research facilities and favorable orphan drug policies, and Europe and Asia-Pacific are also proving to be the high potential areas of expansion. Firms are also taking advantage of hospital and research partnerships as well as AI-based solutions to target discovery and design of patient-specific therapies to improve treatment effectiveness and safety. Viral vectors are leading because they have been demonstrated to be efficient and CRISPR-based therapies and non-viral systems are on the rise. Altogether, there exist tremendous opportunities in the market in relation to precision therapies, accelerated regulatory pathways, and enhanced patient outcomes in the management of ultra-rare diseases.
"Key Driver, Restraint, and Growth Opportunity Shaping the Global Gene Therapy for Ultra-Rare Diseases Market"
Growth in the market is being driven by the increasing incidence of ultra-rare genetic disorders and the patient demand of effective therapies. Long-term effects in therapy can be achieved by the use of advances in platforms of gene delivery such as viral vectors and highly specific gene-editing technologies. Additional encouragement of innovation and commercialization comes through government support, orphan drug development funding, and friendlier regulatory routes, allowing biotechnology firms to create therapies that fulfill unmet medical needs in ultra-rare disease populations around the globe.
Gene therapies are not accessible or adopted due to the high cost of development and treatment. The viral vectors and custom-made treatment are complicated production procedures demanding advanced infrastructure and expertise. Also, strict regulatory needs, possible immune reactions and safety issues in the long-term limit widespread implementation. The small numbers of patients with ultra-rare diseases pose a commercial challenge, especially in emerging markets, slowing the broad adoption despite the advancement of technologies and the increased clinical demand.
The growing interest on the combination therapies opens opportunities to improve the efficacy of gene therapies in ultra-rare diseases. Through combination of gene therapy and supportive pharmacological therapy or immunomodulatory therapy, companies are able to enhance patient outcomes and expand clinical applicability. Partnerships with pharmaceutical companies and clinical research institutes help to develop and receive regulatory approval, which allows manufacturers to increase market penetration and treat intricate ultra-rare diseases more efficiently.
To know more about the Gene Therapy for Ultra-Rare Diseases Market - Download our Sample Report: https://marketgenics.co/download-report-sample/gene-therapy-for-ultra-rare-diseases-market-76271
Expansion of Global Gene Therapy for Ultra-Rare Diseases Market
"Innovation, and public funding propel the global gene therapy for ultra-rare diseases market expansion"
The market of gene therapy of ultra-rare diseases is experiencing a spectacular worldwide growth, which is mainly driven by the continuous development of the viral and non-viral systems of gene delivery, CRISPR, and other innovative technologies of gene-editing. These have been combined with personalized medicine strategies and targeted development of therapeutic solutions and are making possible effective management of previously incurable ultra-rare genetic diseases as well as improving patient-specific safety, efficacy, and long-term clinical outcomes. As an example, VG901 (AAV-based gene therapy against retinitis pigmentosa), an ultra-rare disease gene therapy candidate by ViGeneron GmbH was granted a U.S. Food and Drug Administration Rare Pediatric Disease Designation, which grants them the opportunity to expedite the review procedure and receive a priority status in this ultra-rare ocular disease.
Government and insurance incentives can significantly facilitate the adoption of gene therapy on ultra-rare diseases, since they are significant in reducing the high cost burden of the new types of treatment. An example is the U.S. Programs like the Orphan Drug Act offer tax breaks, funding as well as seven-year market exclusivity upon approval of an approved therapy to motivate firms to invest in research and development of conditions with very limited patient populations. Also, Ultragenyx became a member of the FDA and NIH Bespoke Gene Therapy Consortium (BGTC), a five-year and $76.5 million effort to bring pace to the creation of gene therapies to treat rare diseases. This is a public-private collaboration aimed at maximizing adeno-associated virus (AAV) vectors production and simplifying regulatory approval, respectively, to lower costs and time to market of treatments of ultra-rare conditions.
Regional Analysis of Global Gene Therapy for Ultra-Rare Diseases Market
The market that has the best opportunities is the gene therapy for ultra-rare diseases in North America because of the developed biotechnology ecosystem, the strong clinical research base, and the supportive regulatory climate. The orphan drug incentives by the U.S. Food and Drug Administration, the robust federal and private sector investments, promote innovation and early development. Top organizations like the NIH and the Center of Biologics Evaluation and Research of FDA also expand translational research, making breakthrough therapies in ultra-rare genetic disease more quickly approved and commercialized.
The Asia-Pacific, particularly the gene therapy for ultra-rare diseases market, will experience the best growth due to the emerging global healthcare infrastructure, the growing government efforts to fund research on rare diseases and the increasing number of Indian biotech-pharmaceutical collaboration projects on a global scale. Japan, China, and South Korea are among the countries that have been putting large investments in genomic medicine, CRISPR studies, and domestic production of viral vectors. Likewise, favourable regulatory changes and patient registries are boosting clinical trials approvals and market access. The increasing awareness, diagnostic capacity, and development of rare disease focal centers also make Asia-Pacific a prime growth centre in respect to developing advanced gene therapies.
Prominent players operating in the global gene therapy for ultra-rare diseases market are BioMarin Pharmaceutical Inc., Bluebird bio, Inc., Bristol Myers Squibb Company, CRISPR Therapeutics AG, CSL Behring, Editas Medicine, Inc., Intellia Therapeutics, Inc., Novartis AG, Orchard Therapeutics plc, Passage Bio, Inc., Pfizer Inc., Roche Holding AG, Sangamo Therapeutics, Inc., Sarepta Therapeutics, Inc., Spark Therapeutics (Roche), Takeda Pharmaceutical Company Limited, Ultragenyx Pharmaceutical Inc., uniQure N.V., Vertex Pharmaceuticals Incorporated, and Other Key Players.
Buy Now: https://marketgenics.co/buy/gene-therapy-for-ultra-rare-diseases-market-76271
The global Gene Therapy for Ultra-Rare Diseases market has been segmented as follows:
Global Gene Therapy for Ultra-Rare Diseases Market Analysis, By Vector Type
Viral Vectors
Adeno-Associated Virus (AAV) Vectors
Lentiviral Vectors
Retroviral Vectors
Adenoviral Vectors
Herpes Simplex Virus (HSV) Vectors
Others
Non-Viral Vectors
Plasmid DNA
Lipid Nanoparticles
Electroporation-based
Naked DNA
Others
Global Gene Therapy for Ultra-Rare Diseases Market Analysis, By Disease Type
Neurological Disorders
Spinal Muscular Atrophy (SMA)
Duchenne Muscular Dystrophy (DMD)
Huntington's Disease
Rett Syndrome
Batten Disease
Others
Hematological Disorders
Hemophilia A
Hemophilia B
Sickle Cell Disease
Beta-Thalassemia
Others
Metabolic Disorders
Lysosomal Storage Diseases
Gaucher Disease
Fabry Disease
Pompe Disease
Others
Ophthalmological Disorders
Leber Congenital Amaurosis
Retinitis Pigmentosa
Choroideremia
Others
Immunological Disorders
Cardiovascular Disorders
Others
Global Gene Therapy for Ultra-Rare Diseases Market Analysis, By Therapy Type
In Vivo Gene Therapy
Directly Administered
Systemically Delivered
Others
Ex Vivo Gene Therapy
Cell-based Therapies
Tissue-engineered Products
Others
Gene Editing Therapies
CRISPR/Cas9
TALENs
Zinc Finger Nucleases
Others
Global Gene Therapy for Ultra-Rare Diseases Market Analysis, By Route of Administration
Intravenous (IV)
Intramuscular (IM)
Intrathecal
Subretinal
Intravitreal
Direct Tissue Injection
Global Gene Therapy for Ultra-Rare Diseases Market Analysis, By Age Group
Pediatric Patients
Neonates (0-1 month)
Infants (1 month - 2 years)
Children (2-12 years)
Adolescents (12-18 years)
Adult Patients
Young Adults (18-40 years)
Middle-aged Adults (40-65 years)
Elderly (65+ years)
Global Gene Therapy for Ultra-Rare Diseases Market Analysis, By Product Stage
Approved Therapies
Clinical Stage
Phase I
Phase II
Phase III
Preclinical Stage
Research & Discovery Stage
Global Gene Therapy for Ultra-Rare Diseases Market Analysis, By Technology Platform
AAV-based Platform
Lentiviral Platform
CRISPR-based Platform
Base Editing Platform
Prime Editing Platform
RNA Interference (RNAi)
Antisense Oligonucleotides
Global Gene Therapy for Ultra-Rare Diseases Market Analysis, By Manufacturing Process
Autologous Manufacturing
Allogeneic Manufacturing
Contract Manufacturing
In-house Manufacturing
Global Gene Therapy for Ultra-Rare Diseases Market Analysis, by End Use Industry
Hospitals & Clinics
Diagnostic Services
Treatment Administration
Patient Monitoring
Acute Care Management
Others
Specialty Treatment Centers
Gene Therapy Administration
Post-treatment Care
Clinical Trial Conduct
Patient Follow-up Programs
Others
Research & Academic Institutions
Clinical Research
Drug Development
Biomarker Discovery
Preclinical Studies
Others
Biotechnology & Pharmaceutical Companies
Product Development
Manufacturing & Production
Quality Control
Regulatory Compliance
Others
Contract Research Organizations (CROs)
Clinical Trial Management
Data Analysis
Regulatory Support
Patient Recruitment
Others
Gene Therapy Manufacturing Facilities
Vector Production
Cell Processing
Quality Assurance
Supply Chain Management
Others
Global Gene Therapy for Ultra-Rare Diseases Market Analysis, By Region
North America
Europe
Asia Pacific
Middle East
Africa
South America
About Us
MarketGenics is a global market research and management consulting company empowering decision makers across healthcare, technology, and policy domains. Our mission is to deliver granular market intelligence combined with strategic foresight to accelerate sustainable growth.
We support clients across strategy development, product innovation, healthcare infrastructure, and digital transformation.
Contact:
Mr. Debashish Roy
MarketGenics India Pvt. Ltd.
800 N King Street, Suite 304 #4208, Wilmington, DE 19801, United States
USA: +1 (302) 303-2617
Email: sales@marketgenics.co
Website: https://marketgenics.co
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Gene Therapy for Ultra-Rare Diseases Market Forecast 2035 | Key Driver, Restraint, and Growth Opportunity here
News-ID: 4256937 • Views: …
More Releases from MarketGenics India Pvt. Ltd.
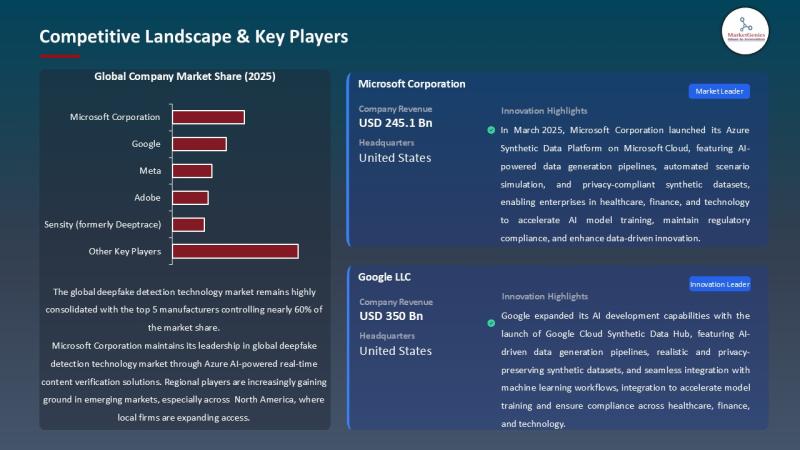
APAC Deepfake Detection Market Accelerates as Governments Tighten Digital Trust …
A Market Transforming How the World Verifies Reality
The global deepfake detection technology market, valued at USD 0.6 billion in 2025, is positioned to accelerate at a powerful 37.2% CAGR, reaching USD 15.1 billion by 2035.
This growth is driven by one undeniable truth:
Synthetic media is reshaping the threat landscape faster than humans can recognize it.
Deepfake detection technologies now determine:
How newsrooms verify breaking content
How financial institutions prevent identity-spoofing
How governments protect election integrity
How…
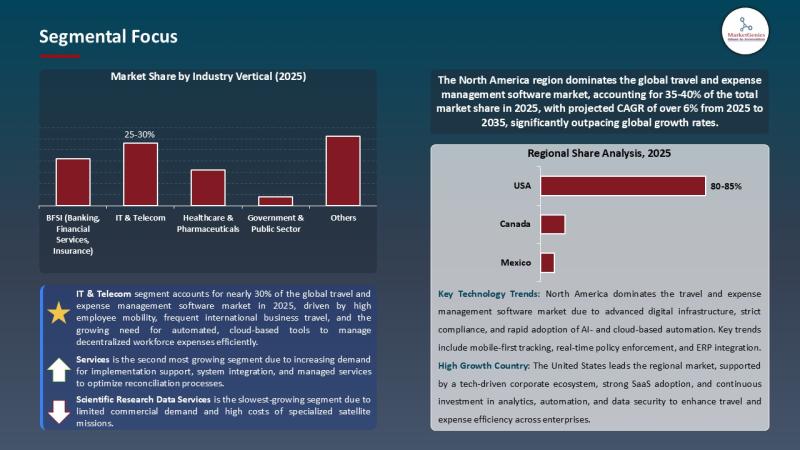
Travel & Expense Management Software Market Signals a Digital Pivot | AI, Cloud …
The Travel and Expense Management (TEM) Market Crossroads | A Sector Accelerating, Repricing Efficiency, and Redrawing the Corporate Spend Map
(Is TEM a Back-Office Tool-or the Operating System of the Next Enterprise Economy?)
For years, the travel and expense management software market lived in the administrative shadows-handed off to finance teams, constrained by spreadsheets, and dismissed as a routine cost-control tool. But the numbers now tell a radically different story.
In 2025, the…
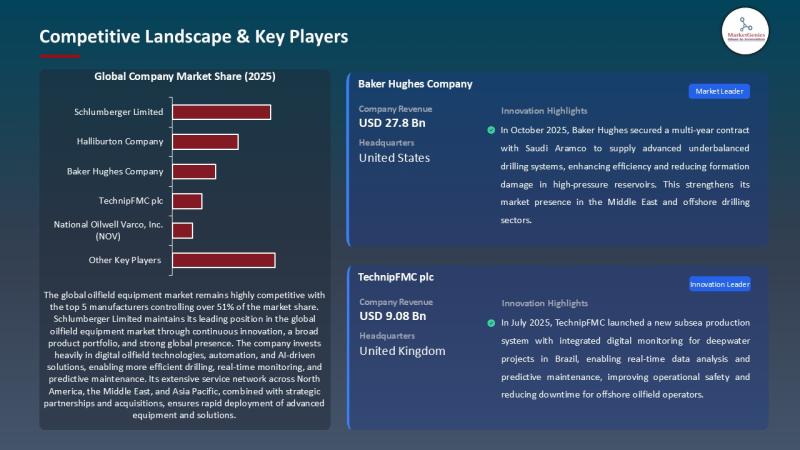
Oilfield Equipment Market hits USD 116.2B in 2025 and grows to USD 156.5B by 203 …
Oilfield Equipment Market | The $156.5B Hardware Backbone of the Global Energy System
Every headline loves clean energy. Yet the global energy mix still demands a brutal truth: oil and gas remain the world's primary supply of heat, mobility, and petrochemicals - and the machines that drill, lift, complete, and produce hydrocarbons continue to define industrial capability.
That's why the Oilfield Equipment Market remains a strategic industry - not a relic.
In 2025,…

Machine Tools Market 2025-2035 | USD 109.9B Growth, CNC & Automation Trends
Machine Tools Market | The $109.9B Intelligence Engine of Global Manufacturing
Factories don't work without machine tools. They shape, cut, drill, grind, and define the physical world around us. Yet most end-products - cars, aircraft parts, electronics housings, surgical devices - never reveal the precision machinery behind them.
The Machine Tools Market is the invisible infrastructure that turns digital models into physical reality.
In 2025, the global Machine Tools Market stands at USD…
More Releases for Disease
Protheragen Announces Disease Model Development Services to Enhance Rare Disease …
Protheragen announced the release of its disease model development services provided to enhance rare disease research. With an increasing focus on rare diseases and the need for tailored research models, Protheragen is dedicated to providing cutting-edge solutions to support this important area of medical research.
Rare diseases, which affect a small percentage of the population, pose unique challenges for researchers due to their limited understanding and lack of available treatment options.…
Biopharmaceutical Fermentation Market 2022 | Growing Morbidity of Genetic Diseas …
According to Precision Business Insights (PBI), the latest report, the biopharmaceutical fermentation market is expected to be valued at USD 10,302.1 million in 2022, growing at a 4.7% CAGR from 2022 to 2028. The growing prevalence of chronic illness, cancer, cardiac diseases, infectious diseases, antibiotic resistance, autoimmune diseases, etc., globally causes the leaning toward biopharmaceutical products, which show promising results in the research studies. Moreover, new findings by the…
Global Inflammatory Bowel Disease (IBD) Market Key Players Analysis, Disease Ind …
Global Inflammatory Bowel Disease (IBD) Market, valued at USD 210.5 Billion in the year 2020 has been driven by factors such as the increasing prevalence of Ulcerative Colitis and Crohn’s Disease, advances in medical technology, government support for inflammatory bowel disease treatment research, and the ubiquity of anxiety and depression. Moreover, factors such as the irregular food habits, unhealthy lifestyle of people, increased level of pollution and increase in alcohol…
Moyamoya Disease Market - A Scientometric Assessment of Disease During 2018-23
Market Highlight:
Moyamoya disease (MMD) is a chronic, cerebrovascular disease characterized by progressive stenosis in the internal carotid artery and an abnormal vascular network at the base of the brain. The stroke is one of the significant symptoms of moyamoya disease, which further leads to paralysis of the face, arms, and legs, loss of speech, and temporary loss of neurologic function of body parts, visual disturbances, headaches, developmental delay, and…
Refsum Disease Industry An Overview of A New Disease - 2018
"Refsum Disease" is a genetic disorder which is inherited as an autosomal recessive trait. The condition is characterized by progressive loss of vision (retinitis pigmentosa), failure of muscle coordination (ataxia), degenerative nerve disease (peripheral neuropathy), and dry, rough, scaly skin (ichthyosis). The accumulation of fatty acid (phytanic acid) in blood plasma and tissues is the main cause of refsum disease. This occurs due to malfunction of the enzyme producing gene…
Chagas Disease (Infectious Disease) Pipeline Forecast Report, H1, 2017 [MRH]
Market Research Hub's Pharmaceutical and Healthcare latest pipeline guide Chagas Disease - Pipeline Review, H1 2017, provides comprehensive information on the therapeutics under development for Chagas Disease Market (Infectious Disease), complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA) and molecule type. The guide covers the descriptive pharmacological action of the therapeutics, its complete research and development history and latest news and…