Press release
Global Orphan Drugs Market to reach USD 357.26 billion growing at a CAGR of 6.98% by 2032, Evaluates DelveInsight
According to DelveInsight's analysis, The orphan drugs market is seeing strong expansion, largely due to the increasing number of rare and genetic diseases worldwide and the significant unmet need for effective treatments. Since many rare conditions still lack approved therapies, the market presents major growth potential. Supportive government regulations-such as incentives, market exclusivity, and faster approval processes-are also encouraging more companies to invest in orphan drug development by lowering financial and regulatory hurdles. Alongside this, rising R&D spending and a surge in new product launches, especially in areas like biotechnology, gene therapy, and personalized medicine, are accelerating innovation. Together, these trends are expected to sustain the market's upward trajectory throughout the 2025-2032 forecast period.DelveInsight's "Orphan Drugs Market Insights, Competitive Landscape and Market Forecast-2032" report provides the current and forecast market outlook, forthcoming device innovation, challenges, market drivers and barriers. The report also covers the major emerging products and key Orphan Drugs companies actively working in the market.
To know more about why North America is leading the market growth in the Orphan Drugs market, get a snapshot of the report Orphan Drugs Market Trends
https://www.delveinsight.com/sample-request/orphan-drugs-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Orphan Drugs Overview
Orphan drugs are medications developed to treat rare diseases or conditions that affect a very small percentage of the population, often referred to as "orphan" or rare diseases. Because the patient population is limited, these drugs are typically not financially attractive for pharmaceutical companies to develop without special incentives. To encourage research and development, governments offer benefits such as market exclusivity, tax credits, and fast-track approvals under regulations like the Orphan Drug Act (1983) in the US and similar policies in the EU.
Orphan drugs play a crucial role in addressing unmet medical needs in fields such as genetic disorders, rare cancers, metabolic diseases, and neurological conditions.
DelveInsight Analysis: The global orphan drugs market size was valued at USD 208.61 billion in 2024 and is expected to expand at a CAGR of 6.98% from 2025 to 2032, ultimately reaching around USD 357.26 billion by 2032.
Orphan Drugs Market Insights
Geographically, North America is expected to retain its leading position in the orphan drugs market in 2024, supported by several key factors. The rising incidence of rare and genetic diseases in the region is driving a strong need for specialized treatments. Government support, especially through the Orphan Drug Act of 1983-which provides incentives such as tax benefits, funding assistance, and market exclusivity-has played a major role in encouraging drug development. Moreover, continuous advancements in biotechnology, higher investments in R&D, and frequent new product approvals from major pharmaceutical companies are further accelerating market growth and expanding therapeutic options for patients with rare conditions that currently lack effective treatments.
To read more about the latest highlights related to Orphan Drugs, get a snapshot of the key highlights entailed in the Orphan Drugs Market Insights
https://www.delveinsight.com/report-store/orphan-drugs-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Recent Developments in the Orphan Drugs Market Report
• In January 2025, Johnson & Johnson announced that its Nipocalimab Biologics License Application (BLA) received Priority Review designation from the U.S. Food and Drug Administration (FDA) for the treatment of antibody-positive generalized myasthenia gravis (gMG) patients. The drug had previously been granted Orphan Drug Designation by both the FDA and European Medicines Agency (EMA).
• In January 2025, NMD Pharma A/S revealed that the FDA has granted Orphan Drug Designation (ODD) to NMD670, a novel oral small-molecule inhibitor targeting the skeletal muscle-specific chloride ion channel ClC-1, for the treatment of Charcot-Marie-Tooth disease (CMT).
• In January 2025, Elicera Therapeutics AB (publ), a clinical-stage cell and gene therapy company, announced that its drug candidate ELC-100 has received Orphan Drug Designation from the FDA for the treatment of pancreatic neuroendocrine tumors. This designation provides substantial regulatory benefits during the continued development and potential marketing approval of the treatment.
• In January 2025, KalVista Pharmaceuticals, Inc. reported that Japan's Ministry of Health, Labour and Welfare (MHLW) granted Sebetralstat Orphan Drug Designation for the on-demand treatment of hereditary angioedema (HAE) attacks in adults and adolescents aged 12 years and older.
• In October 2024, Astellas Pharma Inc. received FDA approval for VYLOYTM (zolbetuximab-clzb), a first-line treatment for HER2-negative gastric or gastroesophageal junction adenocarcinoma.
• Thus, owing to such developments in the market, rapid growth will be observed in the Orphan Drugs market during the forecast period
Key Players in the Orphan Drugs Market
Some of the key market players operating in the Orphan Drugs market include- Sanofi, Biogen, Ionis Pharmaceuticals, Inc., Novartis AG, Alnylam Pharmaceuticals, Inc., F. Hoffmann-La Roche Ltd., AstraZeneca, Gilead Sciences, Inc., Johnson & Johnson Services, Inc., Mitsubishi Tanabe Pharma Corporation, BioMarin Pharmaceutical Inc., Vertex Pharmaceuticals Incorporated, Ultragenyx Pharmaceutical Inc., Kyowa Kirin Co., Ltd., Catalyst Pharmaceuticals, Inc., Chugai Pharmaceutical Co., Ltd., Sarepta Therapeutics, Inc., Astellas Pharma Inc., Pfizer Inc., Novo Nordisk A/S, and others.
Which MedTech key players in the Orphan Drugs market are set to emerge as the trendsetter explore @ Key Orphan Drugs Companies
https://www.delveinsight.com/sample-request/orphan-drugs-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Analysis on the Orphan Drugs Market Landscape
Faster regulatory processes, such as fast-track and accelerated approvals, along with incentives provided under the Orphan Drug Act, are helping advance the development of biologics for rare diseases. These supportive measures are increasing demand and motivating major companies to create more targeted therapies in the orphan drug space. For instance, in March 2024, Cabaletta Bio, Inc. received FDA Orphan Drug Designation for CABA-201, a CD19-CAR T cell therapy being developed for systemic sclerosis (SSc). Likewise, in June 2024, Be Biopharma, Inc. was granted Orphan Drug Designation for BE-101, an engineered B cell therapy for Hemophilia B.
Overall, these developments are expected to strengthen this market segment and contribute to the continued expansion of the orphan drugs market during the forecast period.
Scope of the Orphan Drugs Market Report
• Coverage: Global
• Study Period: 2022-2032
• Orphan Drugs Market Segmentation By Type: Small Molecule and Biologics
• Orphan Drugs Market Segmentation By Route of Administration: Oral and Parenteral
• Orphan Drugs Market Segmentation By Indication: Oncology, Hematology, Ophthalmology, Neurology, and Others
• Orphan Drugs Market Segmentation By Distribution Channel: Hospital and Retail Pharmacies and Online Pharmacies
• Orphan Drugs Market Segmentation By Geography: North America, Europe, Asia-Pacific, and Rest of the World
• Key Orphan Drugs Companies: Sanofi, Biogen, Ionis Pharmaceuticals, Inc., Novartis AG, Alnylam Pharmaceuticals, Inc., F. Hoffmann-La Roche Ltd., AstraZeneca, Gilead Sciences, Inc., Johnson & Johnson Services, Inc., Mitsubishi Tanabe Pharma Corporation, BioMarin Pharmaceutical Inc., Vertex Pharmaceuticals Incorporated, Ultragenyx Pharmaceutical Inc., Kyowa Kirin Co., Ltd., Catalyst Pharmaceuticals, Inc., Chugai Pharmaceutical Co., Ltd., Sarepta Therapeutics, Inc., Astellas Pharma Inc., Pfizer Inc., Novo Nordisk A/S, and others
• Porter's Five Forces Analysis, Product Profiles, Case Studies, KOL's Views, Analyst's View
Interested in knowing how the Orphan Drugs market will grow by 2032? Click to get a snapshot of the Orphan Drugs Market Analysis
https://www.delveinsight.com/sample-request/orphan-drugs-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Contact Us
Gaurav Bora
info@delveinsight.com
+14699457679
www.delveinsight.com
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing end-to-end comprehensive solutions to improve their performance.
Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Orphan Drugs Market to reach USD 357.26 billion growing at a CAGR of 6.98% by 2032, Evaluates DelveInsight here
News-ID: 4251619 • Views: …
More Releases from DelveInsight Business Research
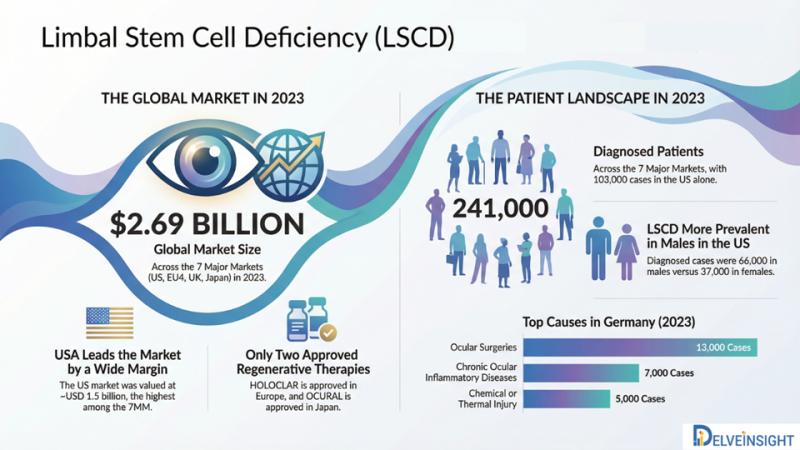
Limbal Stem Cell Deficiency Market to Surpass USD 2.6 Billion by 2034, Driven by …
In 2023, the Limbal Stem Cell Deficiency (LSCD) market was dominated by the United States, generating nearly USD 1.5 billion in revenue, while Spain represented the smallest market with approximately USD 127 million. This regional distribution is expected to remain consistent throughout the forecast timeline. The US accounted for nearly 103,000 diagnosed LSCD cases, whereas Japan recorded around 37,000 cases, with both countries projected to witness notable growth in patient…
SSc-ILD Market Set to Cross USD 750 Million by 2034, Driven by 10+ Emerging Ther …
The major players operating in the Systemic Sclerosis-associated Interstitial Lung Disease (SSc-ILD) market include Roche, Prometheus Biosciences, Inc., Merck, GlaxoSmithKline, Genentech, Inc., Acceleron, Boehringer Ingelheim, Actelion, Hôpital Claude-Huriez, Changchun GeneScience Pharmaceutical, among others.
DelveInsight's report titled "Systemic Sclerosis-associated Interstitial Lung Disease Market Insights, Epidemiology, and Forecast-2034" delivers a comprehensive analysis of SSc-ILD, covering historical data, projected epidemiology, and evolving market trends across the United States, EU4 (Germany, Spain, Italy, and France),…
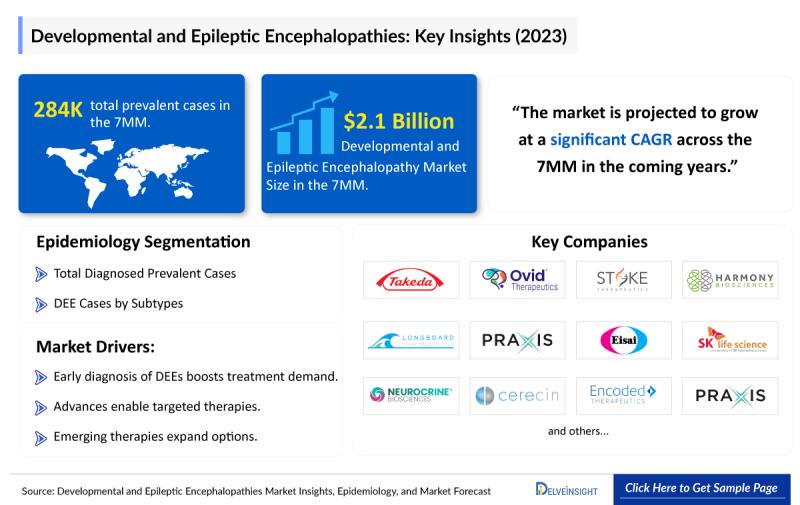
Developmental and Epileptic Encephalopathy Treatment Market Poised for Accelerat …
The Developmental and Epileptic Encephalopathy (DEE) treatment market across the seven major markets (7MM) was valued at nearly USD 2.1 billion in 2023 and is expected to register a healthy compound annual growth rate over the forecast period. The United States emerged as the largest contributor, capturing close to 80% of the overall market revenue.
The DEE treatment landscape is undergoing a significant transformation as the limitations of traditional antiepileptic drugs…
Polycythemia Vera Pipeline and Drug Development in 2025: 10+ Therapies and 8+ Co …
DelveInsight's "Polycythemia Vera Pipeline Insight 2025" report delivers an in-depth overview of the Polycythemia Vera pipeline landscape, covering more than 8 companies and 10+ pipeline candidates. The report analyzes both clinical-stage and preclinical assets, offering detailed drug profiles across various stages of development. It also evaluates Polycythemia Vera therapies based on product classification, development stage, route of administration, and molecular category, while additionally spotlighting inactive or discontinued pipeline assets.
Explore the…
More Releases for Orphan
Acquired Orphan Blood Disease Market
Acquired Orphan Blood Disease Market to reach over USD 18.93 billion by the year 2031 - Exclusive Report by InsightAce Analytic
According to a new report by InsightAce Analytic, the "Acquired Orphan Blood Disease Market" in terms of revenue was estimated to be worth $8.65 billion in 2023 and is poised to reach $18.93 billion by 2031, growing at a CAGR of 10.47% from 2024 to 2031.
Get Free Access to…
Orphan Drugs Market Size to Hit $3199.3 Billion by 2028 | Orphan Drugs Industry …
Market Overview:
According to our experience research team, Orphan Drugs Market was valued at USD 112.36 Billion in 2021, and the global Orphan Drugs industry is projected to reach a value of USD 3199.3 Billion by 2028, at a CAGR of 7.4% during the forecast period 2022-2028
Vantage Market Research is a collection of market research studies on several industries, such as Chemicals, semiconductors & Electronics, Food & Beverages Technology, Energy &…
Orphan Drugs for Cancer Pipeline Analysis
A huge market opportunity is offered by small patient population which suffers from rare or orphan diseases. Among the category of new orphan drugs, Oncology account for the largest disease group in recent years. It has been observed that majority of the orphan drugs in the clinical stages are for rare cancer disease drugs, and are in the late stages of the pipeline. Some of the drugs are being developed…
US Orphan Drug Pipeline Analysis
In recent years, the pharmaceutical industry has been experiencing a paradigm shift. While a large pool of patients was considered as a major source of revenue for pharma companies in the past, the focus is now gradually shifting to small sections of patients suffering from rare disease. In US, this pool of patients is gradually growing and orphan drugs are becoming an extremely attractive business proposition for the pharmaceuticals industry.…
Europe Orphan Drugs Pipeline Analysis
“Europe Orphan Drugs Pipeline Analysis” by PNS Pharma gives comprehensive insight on the various drug profiles under Orphan Drugs status in Europe. Research report covers all the ongoing drug development in various phases. Each drug profiles include detailed information like: Originator, Owner, Collaborator, Technology Provider, Licensee, Development Phase, Development Indications, Mechanism of Action, Chemical Formula, Country of Development and detailed analysis on the development process. The information for particular drug…
Global Orphan Drug Pipeline Analysis
In recent years, the pharmaceutical industry has been experiencing a paradigm shift. While a large pool of patients was considered as a major source of revenue for pharma companies in the past, the focus is now gradually shifting to small sections of patients suffering from rare disease. In US & Europe, this pool of patients is gradually growing and orphan drugs are becoming an extremely attractive business proposition for the…
