Press release
GMP Storage Market to Witness Steady Growth Driven by Biopharma Expansion - Persistence Market Research
The global Good Manufacturing Practice (GMP) Storage Market has evolved into a cornerstone of the modern pharmaceutical and biotechnology industries. As of 2021, the worldwide GMP storage market was valued at approximately US$ 5.7 billion, with projections indicating a robust expansion at a compound annual growth rate (CAGR) of 5.6%, expected to reach US$ 10.2 billion by 2032. This impressive trajectory underscores the indispensable role of GMP-compliant storage facilities in ensuring the integrity, stability, and regulatory compliance of temperature-sensitive biologics, vaccines, and pharmaceuticals.The increasing focus on maintaining stringent quality standards for biologics and cell-based therapies has driven the adoption of temperature-controlled GMP storage solutions across the pharmaceutical supply chain. The global surge in vaccine production-particularly following the COVID-19 pandemic-has further accelerated demand for advanced, reliable, and regulatory-approved storage infrastructure. Among the various market segments, GMP storage products represent the leading category, forecasted to reach a value of US$ 8.3 billion by 2032. Geographically, North America dominates the market, accounting for the majority share, led by the United States due to its high volume of FDA-approved temperature-sensitive drugs and strong pharmaceutical manufacturing base.
Get a Sample Copy of Research Report (Use Corporate Mail id for Quick Response): https://www.persistencemarketresearch.com/samples/33113
Market Statistics and Growth Drivers
Between 2012 and 2021, the GMP storage market expanded at a historic CAGR of 4.4%, reflecting steady growth driven by the rising complexity of biopharmaceutical manufacturing. The subsequent decade is expected to witness even stronger growth due to new regulatory frameworks, advanced storage technologies, and the increasing global footprint of biologics and gene therapies.
Key growth drivers include the expansion of biologics manufacturing, rising investments in cell and gene therapy production, and the outsourcing of GMP storage services by pharmaceutical companies seeking flexibility and scalability. Furthermore, the integration of digital monitoring systems, energy-efficient refrigeration technologies, and cloud-based data management has made temperature-controlled logistics more secure and efficient. As companies aim to comply with international GMP standards, the need for specialized, cost-effective storage solutions has become central to their operations, pushing the market toward innovation and consolidation.
Key Highlights from the Report
• Global GMP storage market valued at US$ 5.9 billion in 2022, projected to reach US$ 10.2 billion by 2032.
• The market is anticipated to grow at a CAGR of 5.6% between 2022 and 2032.
• Top five countries collectively account for 69% of global market share.
• GMP storage products constitute nearly 83% of total market revenues.
• North America leads globally, with the U.S. representing 89% of the regional share.
• Increasing regulatory stringency and growth of biologics and cell therapies drive sustained demand.
Market Segmentation Analysis
The GMP storage market segmentation reflects the diversity of products, services, and end-user requirements across the life sciences ecosystem.
By Product Type, the market is broadly divided into GMP storage products and GMP storage services. Among these, products-including biomedical refrigerators, freezers, and temperature-controlled storage systems-dominate with around 83.1% share in 2021. This dominance is attributed to the rising number of biologic products requiring strict temperature maintenance, coupled with the development of high-performance refrigeration technologies by manufacturers such as ThermoFisher Scientific and Eppendorf AG.
By Application, the biologics segment leads with approximately 38% of total market share. This category includes vaccines, plasma, and cell-based products that are inherently temperature-sensitive and demand consistent storage conditions to maintain efficacy. The shift toward personalized medicine and regenerative therapies further bolsters this segment's growth.
By End User, biopharmaceutical companies accounted for the largest portion, representing 42.5% of total revenues in 2021. These organizations often rely on outsourced GMP storage services to ensure global regulatory compliance and cost efficiency. The trend toward contract-based storage and logistics solutions is particularly strong among mid-sized pharma firms, allowing them to maintain focus on R&D while reducing infrastructure expenditure.
Read Detailed Analysis: https://www.persistencemarketresearch.com/market-research/gmp-storage-market.asp
Regional Insights
Geographically, the GMP storage market demonstrates distinct regional patterns driven by local industry capabilities, regulatory frameworks, and infrastructure development.
North America, led by the United States, is the dominant regional market. The U.S. accounted for more than 89% of the North American GMP storage share in 2021, driven by increasing FDA approvals for temperature-sensitive drugs. Nearly half of all new drug approvals in recent years required refrigerated or frozen storage, reflecting the growing prevalence of biologics in clinical and commercial pipelines. Furthermore, regulatory flexibility-such as extended refrigerator storage periods for thawed vaccines-has intensified demand for reliable GMP-compliant storage infrastructure across pharmaceutical and healthcare sectors.
Europe, with Germany as its key growth hub, remains a lucrative market owing to its strong biopharmaceutical manufacturing base and advanced regulatory environment. Germany's BioPharma Cluster South stands as one of the world's leading centers for biotechnological innovation, generating over €4.5 billion in dedicated biotechnology revenues. The country's focus on traceable, compliant cold-chain supply systems reinforces its leadership in the European GMP storage sector.
In East Asia, China commands nearly half of the regional market (49.4%), supported by massive government investments in biotech R&D and infrastructure. The creation of over 600 biotech science parks and innovation hubs highlights China's drive toward self-sufficiency and large-scale biopharmaceutical production. These developments translate directly into heightened demand for GMP storage services for clinical and commercial biologics.
Meanwhile, emerging markets in Latin America and the Middle East are gaining traction as multinational pharmaceutical companies expand their manufacturing bases, creating new opportunities for third-party storage providers and local logistics partners.
Market Drivers
The primary drivers of the GMP storage market stem from the growing complexity of biological products and the increasing need for temperature integrity throughout the supply chain. The rapid growth of biopharmaceuticals, gene therapies, and vaccines has made reliable storage systems critical for maintaining product efficacy.
Another major factor is the outsourcing trend in the pharmaceutical sector. Companies increasingly prefer external GMP storage providers to minimize upfront capital investments while ensuring compliance with international standards. This model enhances operational flexibility, allowing pharmaceutical firms to scale production based on market demand.
Additionally, technological advancements have transformed cold storage solutions. The integration of IoT-enabled monitoring systems, controlled-rate freezers, and energy-efficient compressors has improved temperature regulation, minimized risks of deviation, and reduced operational costs. For example, BioLife Solutions' introduction of high-capacity controlled-rate freezers and SY-LAB's touchscreen-enabled IceCube® systems demonstrate how innovation continues to shape market competitiveness.
Regulatory pressure also plays a vital role in driving market growth. With agencies such as the U.S. FDA and European Medicines Agency (EMA) enforcing rigorous standards for biologic storage and distribution, companies are compelled to adopt compliant, state-of-the-art GMP storage infrastructure.
Market Restraints
Despite strong growth prospects, the GMP storage market faces several challenges. The high capital cost associated with building and maintaining temperature-controlled facilities remains a significant barrier, particularly for small and medium-sized enterprises (SMEs). Establishing a GMP-compliant storage unit requires substantial investment in refrigeration systems, backup power, security infrastructure, and continuous monitoring systems.
Additionally, rising energy costs have become a major operational concern. Reports indicate that electricity expenses can consume up to 30% of earnings before interest, taxes, depreciation, and amortization (EBITDA) for some cold storage businesses. Maintaining consistent power supply and temperature conditions further escalates costs, especially in regions with unstable grids.
Another limitation is the complex regulatory landscape, where compliance requirements differ from country to country. The variation in standards creates logistical hurdles for multinational operations, often necessitating separate validation processes for each market. Furthermore, a shortage of skilled professionals trained in GMP protocols and facility management adds another layer of difficulty in scaling operations globally.
Market Opportunities
The coming decade presents significant opportunities for innovation and expansion in the GMP storage market. The growing demand for cell and gene therapies, combined with increasing clinical trial volumes, creates immense need for specialized ultra-low temperature and cryogenic storage facilities.
Digitalization and smart monitoring technologies are set to redefine operational efficiency. Cloud-based temperature tracking, real-time deviation alerts, and predictive maintenance systems allow companies to optimize performance while minimizing downtime. This shift also enables greater transparency across the cold chain, facilitating regulatory compliance and quality assurance.
Emerging economies such as India, Brazil, and South Korea are projected to offer lucrative opportunities as global pharmaceutical firms establish local manufacturing hubs. Partnerships between domestic logistics providers and international cold chain specialists are expected to accelerate infrastructure development. Moreover, the sustainability agenda-emphasizing energy efficiency and carbon reduction-will drive innovation in green refrigeration technologies, further enhancing market potential.
Request for Customization of the Research Report: https://www.persistencemarketresearch.com/request-customization/33113
Company Insights
The competitive landscape of the GMP storage market is characterized by product innovation, strategic acquisitions, and geographic expansion. Leading companies are focusing on the development of energy-efficient, digitally integrated storage systems to strengthen their global presence.
Key players operating in the market include:
• ThermoGenesis Holdings, Inc.
• ThermoFisher Scientific Inc.
• BioLife Solutions, Inc.
• Danaher (Cytiva)
• MEDIPOST
• REMI Group
• Eurofins Scientific
• Eppendorf AG
• Intertek Group Plc
• Arctiko
• Bioline Technologies
• Hindustan Apparatus Mfg. Co.
• P L Tandon & Co.
• Stericox India Private Limited
• SY-LAB Geräte GmbH
• BioConvergence LLC (Singota Solutions)
• PHC Holdings Corporation
• Pace Life Sciences
• Haier Biomedical
• Helmer Scientific Inc.
Key Segments Covered in GMP Storage Industry Research
GMP Storage Market by Product & Service:
GMP Storage Products
Refrigerators and Freezers
Cryogenic Storage
GMP Storage Services
GMP Storage Market by Application:
Cell & Gene Therapy
Cell Banking
Biologics
Small Molecules
Others
GMP Storage Market by End User:
Biopharmaceutical Companies
Contract Manufacturing Organizations
Contract Research Organizations
Research & Academic Institutes
GMP Storage Market by Region:
North America GMP Storage Market
Latin America GMP Storage Market
Europe GMP Storage Market
South Asia GMP Storage Market
East Asia GMP Storage Market
Oceania GMP Storage Market
The Middle East & Africa GMP Storage Market
Recent Developments
BioLife Solutions, Inc. acquired Global Cooling, Inc. in May 2021, enhancing its freezer technology portfolio and strengthening its position in the cell therapy supply chain.
In April 2021, BioLife Solutions launched a new high-capacity controlled-rate freezer line, targeting the increasing need for scalable GMP-compliant cold storage for advanced therapy medicinal products (ATMPs).
These developments reflect an industry trend toward technological integration, collaboration, and consolidation aimed at improving service scalability and product reliability.
Conclusion
The GMP Storage Market stands at the intersection of regulatory compliance, technological innovation, and global health needs. As the pharmaceutical landscape shifts toward biologics, vaccines, and cell therapies, the importance of maintaining strict GMP standards across every stage of storage and distribution has never been greater.
While high infrastructure and energy costs continue to challenge profitability, the ongoing advancements in smart storage technology, renewable energy integration, and outsourcing models are creating new pathways for sustainable growth. North America remains the market leader, but emerging regions-particularly in Asia-Pacific-are rapidly building capacity to support their growing biopharmaceutical sectors.
Looking ahead, the GMP storage market is set to evolve beyond traditional refrigeration toward digitally connected, energy-optimized, and compliance-driven ecosystems. With rising investment, innovation, and global collaboration, the sector is poised to reach its projected US$ 10.2 billion valuation by 2032, ensuring the integrity and safety of life-saving biological products for years to come.
Read More Related Reports:
G-Protein Coupled Receptors Market https://www.persistencemarketresearch.com/market-research/g-protein-coupled-receptors-market.asp
CRBSI Treatment Market https://www.persistencemarketresearch.com/market-research/crbsi-treatment-market.asp
Opioid Analgesics Market https://www.persistencemarketresearch.com/market-research/opioid-analgesics-market.asp
Drug Discovery Enzymes Market https://www.persistencemarketresearch.com/market-research/drug-discovery-enzymes-market.asp
Contact Us:
Persistence Market Research
Second Floor, 150 Fleet Street, London, EC4A 2DQ, United Kingdom
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release GMP Storage Market to Witness Steady Growth Driven by Biopharma Expansion - Persistence Market Research here
News-ID: 4250010 • Views: …
More Releases from Persistence Market Research
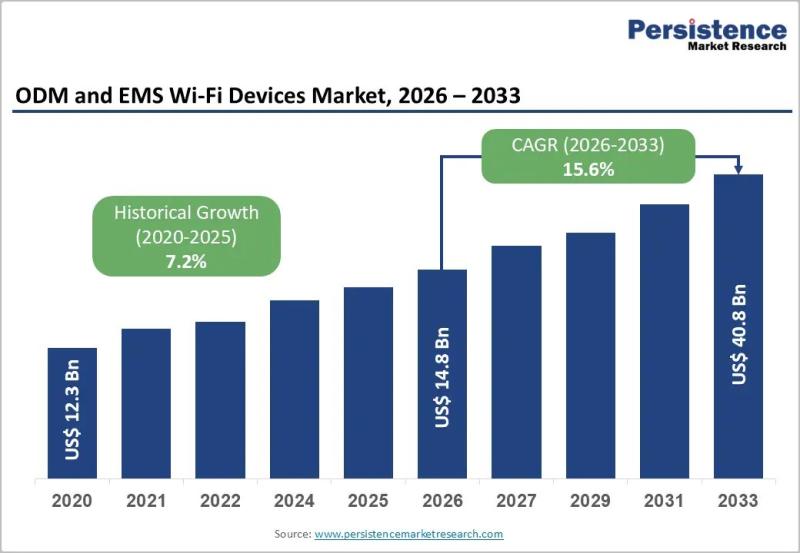
ODM and EMS Wi-Fi Devices Market to Reach US$ 40.8 Billion by 2033 at 15.6% CAGR
The global ODM and EMS Wi-Fi devices market is projected to be valued at US$ 14.8 billion in 2026 and is forecast to surge to US$ 40.8 billion by 2033, registering a robust CAGR of 15.6% between 2026 and 2033. This rapid growth reflects the accelerating demand for advanced wireless connectivity solutions across residential, enterprise, and industrial environments. The expansion of 5G infrastructure, enterprise digital transformation strategies, and large-scale IoT…
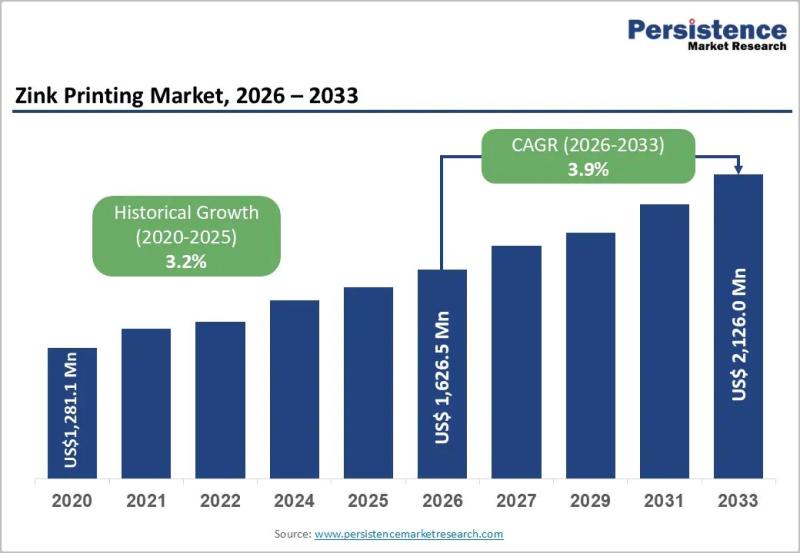
Zink Printing Market to Reach US$ 2,126.0 Million by 2033 at 3.9% CAGR
Zink Printing Market Size and Trends Analysis
The global Zink printing market is projected to be valued at US$ 1,626.5 million in 2026 and is expected to reach US$ 2,126.0 million by 2033, expanding at a CAGR of 3.9% between 2026 and 2033. Zink (Zero Ink) printing technology eliminates the need for ink cartridges by using heat-activated color crystals embedded within specialized paper. This innovation has positioned Zink printers as a…
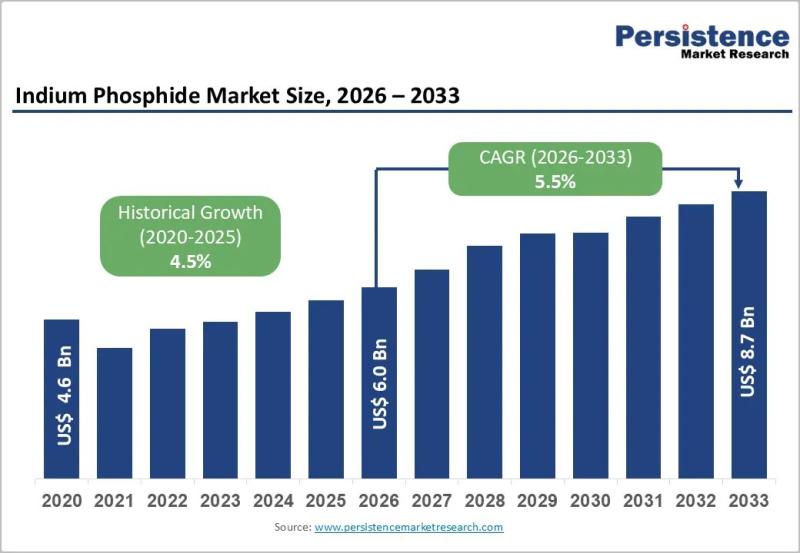
Indium Phosphide Market to Reach US$ 8.7 Billion by 2033 at 5.5% CAGR
The global Indium Phosphide (InP) market is poised for steady expansion, with its valuation expected to reach US$ 6.0 billion by 2026 and further grow to US$ 8.7 billion by 2033, registering a CAGR of 5.5% between 2026 and 2033. Indium phosphide, a high-performance compound semiconductor material, is widely used in optoelectronics, high-frequency electronics, and photonic integrated circuits (PICs). The market growth is largely fueled by the accelerating deployment of…

Roasted Corn Market to Reach $5.5B by 2033 Driven by Rising Snack Demand
The global roasted corn market is poised for steady expansion, driven by rising consumer preference for convenient, ready-to-eat snacks and increasing awareness of healthier alternatives to traditional fried snack options. Current market estimates indicate that the roasted corn market is valued at approximately US$ 3.8 billion in 2026 and is projected to reach US$ 5.5 billion by 2033, reflecting a compound annual growth rate (CAGR) of 5.4% between 2026 and…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…
