Press release
IVD Reagents Market Insights: Global Value to Touch US$ 119.5 Bn by 2032, Finds Persistence Market Research
The global In Vitro Diagnostics (IVD) reagents market is poised for significant expansion, with projections indicating that it will reach a value of approximately USD 77.4 billion by 2025. This market is expected to witness a robust compound annual growth rate (CAGR) of 6.4% from 2025 to 2032, eventually attaining a market value of USD 119.5 billion by 2032. The increasing prevalence of chronic diseases, an aging global population, and rising demand for personalized medicine are some of the key factors propelling the growth of the IVD reagents market.IVD reagents are substances or chemicals used in diagnostic tests that are performed outside the body (in vitro) to detect, measure, or interact with components of biological samples. These reagents are vital in disease detection, monitoring, and treatment. With the continuous advancements in diagnostic technologies, such as molecular diagnostics and point-of-care testing (POCT), the adoption of IVD reagents is witnessing an upward trajectory. Furthermore, the increasing demand for biomarker-based tests, improved healthcare infrastructure, and growing research and development activities are contributing to the market's growth.
Get a Sample Copy of Research Report (Use Corporate Mail id for Quick Response): https://www.persistencemarketresearch.com/samples/35181
Market Statistics and Growth Drivers
The IVD reagents market is expected to exhibit strong growth due to several influential factors. The global market size for IVD reagents is projected to grow at a CAGR of 6.4% from 2025 to 2032, reflecting the increasing adoption of advanced diagnostic techniques and expanding healthcare systems.
Several factors are contributing to the growth of the IVD reagents market, including:
Technological innovations in molecular diagnostics, immunoassays, and next-generation sequencing (NGS).
The growing prevalence of chronic diseases such as diabetes, cancer, and cardiovascular diseases, which are pushing for more frequent and precise diagnostic testing.
The booming biotech sector, with advancements in personalized medicines and genomic testing fueling demand for specialized IVD reagents.
Government initiatives and funding supporting the development and adoption of innovative diagnostic technologies, including point-of-care testing.
The antibodies segment is expected to hold a substantial share of the IVD reagents market in the coming years, with the segment projected to represent about 23.5% of the market by 2025. The growing popularity of antibody-based diagnostic tests is attributed to their high specificity and sensitivity, making them ideal for various diagnostic applications, including cancer detection and infectious disease testing. Furthermore, the clinical laboratories segment is forecasted to dominate the market, accounting for around 35% of the market share by 2025, owing to its established infrastructure, expertise, and adherence to stringent regulatory standards.
In terms of geographical distribution, North America is projected to lead the global market, accounting for approximately 38.7% of the market share in 2025. This is primarily driven by the region's well-established healthcare system, robust diagnostic infrastructure, and increased investments in innovative diagnostic technologies. North America also benefits from ongoing government support for the development and commercialization of advanced IVD products.
Key Highlights from the Report
• Market size expected to reach USD 77.4 billion by 2025, growing at a CAGR of 6.4%.
• Antibodies segment projected to hold 23.5% market share in 2025 due to their high specificity and sensitivity.
• Clinical laboratories expected to lead the market with a 35% share by 2025.
• North America to dominate the IVD reagents market with a 38.7% share by 2025.
• Increased demand for personalized medicine and biomarker-based tests driving market growth.
• Technological innovations such as molecular diagnostics and point-of-care testing are key growth drivers.
Market Segmentation
The IVD reagents market is segmented based on several factors such as reagent type, end-user industry, and geographic location. Understanding these segments helps identify the core areas driving growth and pinpoint the regions where opportunities are likely to expand.
Type of Reagents
IVD reagents are primarily categorized by their chemical composition, which includes antibodies, enzymes, substrates, and others. Among these, the antibodies segment is expected to dominate the market, holding a market share of about 23.5% in 2025. Antibodies are favored in various diagnostic tests due to their high specificity, making them invaluable in detecting diseases such as cancer, diabetes, and infectious conditions.
Antibody-based tests like Enzyme-Linked Immunosorbent Assay (ELISA), chemiluminescence immunoassays, and lateral flow assays are frequently used in diagnosing infectious diseases such as HIV, malaria, and COVID-19. Moreover, antibodies are also playing a significant role in cancer diagnostics by identifying tumor markers, thereby aiding early detection and monitoring the effectiveness of therapies.
End-Use Industry
In terms of end-users, clinical laboratories are expected to account for the largest market share, approximately 35%, by 2025. Clinical laboratories are central to the IVD reagents market due to their capacity for high-throughput testing, advanced infrastructure, and adherence to stringent quality control measures. These laboratories are equipped to handle complex diagnostic tests, which makes them crucial for industries like oncology, infectious diseases, and autoimmune diagnostics.
Moreover, clinical laboratories are increasingly adopting automated systems to enhance testing efficiency and reduce human error, which in turn drives demand for specialized IVD reagents.
Read Detailed Analysis: https://www.persistencemarketresearch.com/market-research/ivd-reagents-market.asp
Regional Insights
North America
North America is expected to lead the global IVD reagents market, with the United States driving the market's growth. Factors contributing to this dominance include a robust healthcare infrastructure, high healthcare expenditure, and increasing investment in diagnostic technologies. The U.S. has seen significant advances in molecular diagnostics, immunoassays, and next-generation sequencing technologies, all of which are supported by government initiatives and funding.
The region's healthcare system also benefits from a high level of automation and adoption of point-of-care testing systems, which further boosts the demand for IVD reagents.
Asia-Pacific
The Asia-Pacific region is also projected to experience substantial growth in the IVD reagents market due to the increasing healthcare investments and the rising demand for diagnostic technologies. Countries like China, India, and Japan are key players in this growth trajectory. The expansion of IVD companies like Sysmex Corporation and Fujifilm in this region is playing a significant role in market expansion.
Innovations such as Loop-mediated Isothermal Amplification (LAMP) technology by Eiken Chemical for rapid disease detection are expected to propel the market further. The rising demand for affordable diagnostics and greater awareness about healthcare is likely to drive adoption of IVD reagents in this region.
Europe
Europe's IVD reagents market is driven by the region's stringent regulatory frameworks and increasing adoption of molecular diagnostics. The implementation of the In Vitro Diagnostic Regulation (IVDR) in Europe has raised the bar for diagnostic products, prompting companies to invest heavily in R&D to meet the new standards. The market is also expanding due to growing demand for personalized medicine, which relies heavily on advanced diagnostic reagents.
Market Drivers
Several factors are driving the growth of the IVD reagents market. The increasing prevalence of chronic diseases such as diabetes, cardiovascular conditions, and cancer is creating a greater demand for diagnostic tests. These diseases require regular monitoring, which boosts the adoption of IVD reagents.
In addition, the shift toward personalized medicine is a significant driver. Personalized diagnostics, including biomarker-based tests and genetic screening, demand highly specific and sensitive reagents. This trend is gaining momentum as more patients seek customized treatments based on their genetic profiles.
Moreover, the ongoing advancements in automation and point-of-care testing (POCT) systems are enhancing the efficiency and accuracy of diagnostic workflows, further propelling the demand for IVD reagents. These technologies help reduce turnaround times and improve patient outcomes, making them highly desirable in both clinical laboratories and remote healthcare settings.
Market Restraints
Despite the promising growth, the IVD reagents market faces several challenges, particularly stringent regulatory requirements. Regulatory authorities such as the FDA in the United States and the National Medical Products Administration (NMPA) in China impose strict guidelines on the development, testing, and commercialization of IVD reagents. These regulations, although necessary to ensure safety and efficacy, can delay market entry and increase operational costs for manufacturers, especially smaller companies.
Another challenge is the high cost of advanced diagnostic technologies. While the demand for highly specialized reagents is growing, the costs associated with developing and manufacturing these reagents can be prohibitive. This can be especially challenging in emerging markets, where healthcare systems may be constrained by budget limitations.
Market Opportunities
The rise of self-testing and home diagnostic procedures presents a significant opportunity for the IVD reagents market. The increasing preference for at-home health monitoring, amplified by the COVID-19 pandemic, is pushing the demand for convenient and easy-to-use diagnostic tests.
Additionally, the integration of AI and IoT in diagnostic systems is expected to enhance the capabilities of IVD reagents, leading to more precise and efficient diagnostics. These advancements open up new growth avenues for companies focused on point-of-care solutions and telemedicine.
Request for Customization of the Research Report: https://www.persistencemarketresearch.com/request-customization/35181
Company Insights
Key players operating in the global IVD reagents market include:
• Abbott Laboratories
• Agilent Technologies
• Becton Dickinson
• bioMérieux
• Bio-Rad Laboratories
• Danaher Corporation
• DiaSorin
• Roche Diagnostics
• Seegene
• Sysmex Corporation
• Thermo Fisher Scientific
• Siemens Healthineers
• Transasia Bio-Medicals Ltd.
• Beacon Diagnostics Pvt. Ltd.
Market Segmentation
By Type of Reagent
Antibodies
Monoclonal Antibodies
Polyclonal Antibodies
Antigens
Oligonucleotides
Enzymes
Nucleic Acid Probes
Others
By Technology
Immunoassay
Molecular Diagnostics
Clinical Chemistry
Hematology
Microbiology
Others
By Application
Infectious Diseases
Cancer/Oncology
Cardiology
Autoimmune Diseases
Genetic Testing
Neurology
Others
By End-Use Industry
Hospitals
Clinical Laboratories
Point-of-Care Testing Facilities
Home Care Settings
Others
By Region
North America
Europe
East Asia
South Asia and Oceania
Latin America
The Middle East and Africa
Recent Developments
In September 2024, CleanNA launched a CE-IVD-compliant version of its CleanNGS product for nucleic acid cleanup in clinical diagnostic settings.
In March 2024, Twist Bioscience released its IVDR-compliant Precision Dx Products, tailored for Whole Exome Sequencing in clinical applications.
Conclusion
The IVD reagents market is poised for robust growth over the next decade, driven by technological innovations, the increasing prevalence of chronic diseases, and a rising demand for personalized medicine. With North America leading the charge, followed by substantial growth in Asia-Pacific and Europe, the market offers significant opportunities for companies investing in next-generation diagnostics. However, challenges such as regulatory complexities and high operational costs remain. As the market continues to evolve, the adoption of point-of-care testing and self-testing procedures is expected to open new avenues for growth, making the future of IVD reagents highly promising.
Read More Related Reports:
Low and Middle Income Countries Opioid Substitution Therapy Market https://www.persistencemarketresearch.com/market-research/low-middle-income-countries-opioid-substitution-therapy-market.asp
Antibody Discovery Market https://www.persistencemarketresearch.com/market-research/antibody-discovery-market.asp
Myasthenia Gravis Treatment Market https://www.persistencemarketresearch.com/market-research/myasthenia-gravis-treatment-market.asp
Earwax Removal Market https://www.persistencemarketresearch.com/market-research/earwax-removal-market.asp
Contact Us:
Persistence Market Research
Second Floor, 150 Fleet Street, London, EC4A 2DQ, United Kingdom
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release IVD Reagents Market Insights: Global Value to Touch US$ 119.5 Bn by 2032, Finds Persistence Market Research here
News-ID: 4247834 • Views: …
More Releases from Persistence Market Research
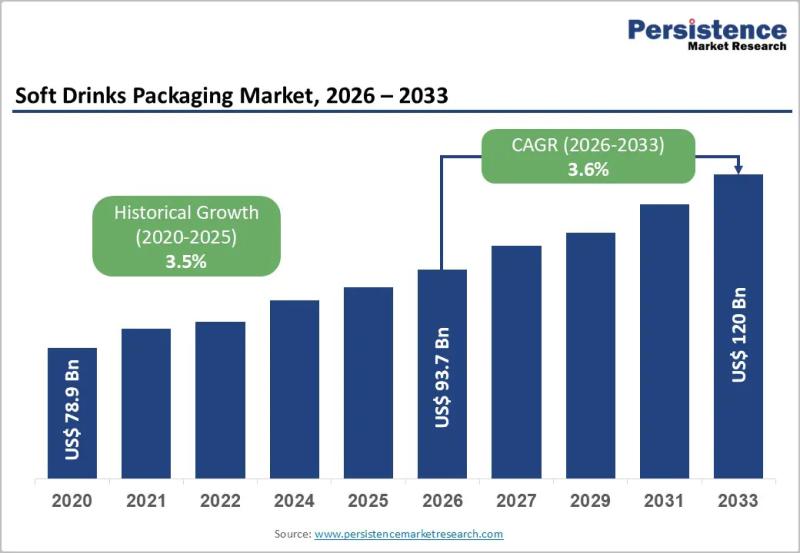
Soft Drinks Packaging Market to Reach US$120.0 Billion by 2033 - Persistence Mar …
The soft drinks packaging market plays a central role in the global beverage industry, serving carbonated drinks, juices, flavored water, energy drinks, and ready to drink teas and coffees. Packaging is no longer limited to containment and transportation; it has evolved into a critical component of branding, sustainability strategy, consumer convenience, and supply chain efficiency. Manufacturers are increasingly focusing on lightweight materials, recyclable packaging formats, and innovative designs that improve…
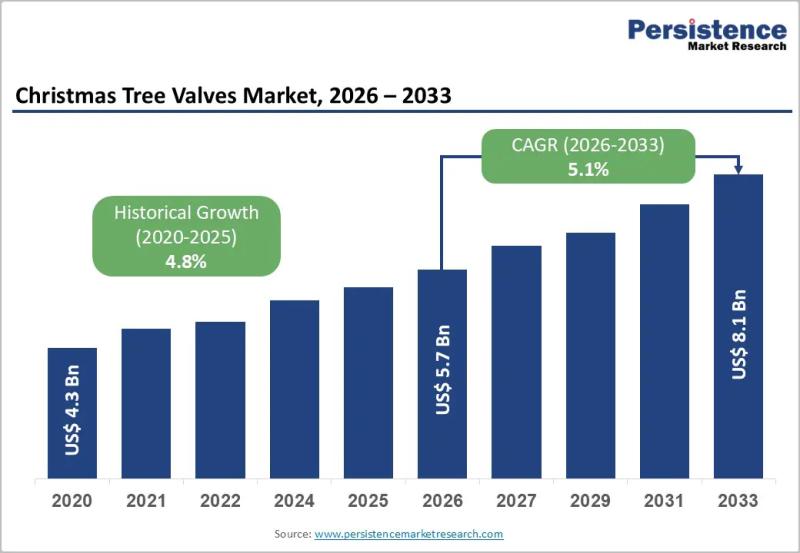
Christmas Tree Valves Market Size to Reach US$8.1 Billion by 2033 - Persistence …
The Christmas Tree Valves Market plays a critical role in the upstream oil and gas industry, serving as a central component in wellhead equipment systems. Christmas tree valves are installed on oil and gas wells to control pressure, regulate flow, and ensure safe extraction of hydrocarbons. These assemblies, commonly referred to as "Christmas trees," consist of multiple valves, spools, and fittings arranged in a structure that resembles a decorated tree.…
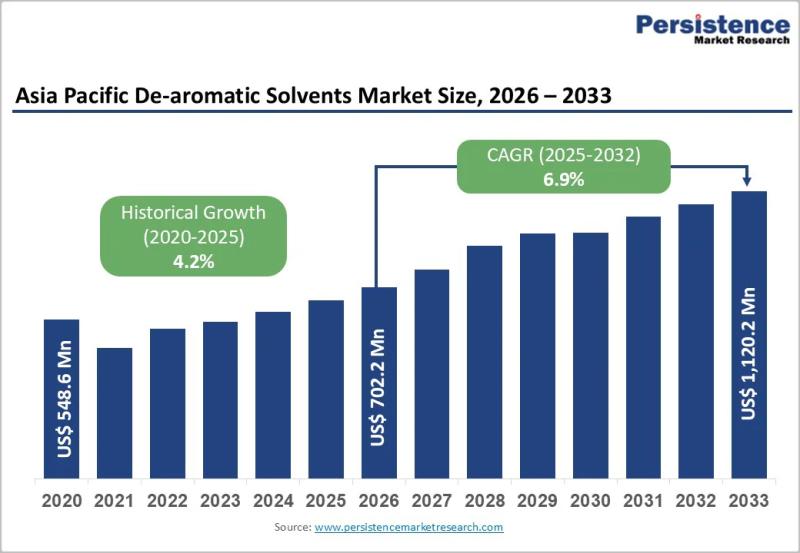
Asia Pacific De-aromatic Solvents Market to Reach US$1,120.2 Million by 2033 - P …
The Asia Pacific De-aromatic Solvents Market is gaining steady momentum as industries across the region increasingly shift toward low aromatic, high purity solvent formulations. De-aromatic solvents are hydrocarbon solvents that have significantly reduced aromatic content, making them suitable for applications requiring low odor, lower toxicity, and improved environmental performance. These solvents are widely used in paints and coatings, adhesives, inks, metalworking fluids, agrochemicals, and cleaning formulations. As regulatory scrutiny around…

Off-Highway Radiators Market to Reach US$ 7.2 Bn by 2033 as Leading Players Like …
The off-highway radiators market plays a vital role in ensuring efficient thermal management in heavy-duty equipment used across construction, agriculture, mining, and forestry sectors. These radiators regulate engine temperatures, prevent overheating, and support consistent equipment performance under extreme operating conditions. Growing mechanization and the expansion of infrastructure projects worldwide are increasing reliance on durable cooling systems. Equipment manufacturers are prioritizing high-performance radiators that offer reliability, longer service life, and resistance…
More Releases for IVD
Transformative Trends Impacting the Cancer In Vitro Diagnostics (IVD) Market Lan …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Cancer In Vitro Diagnostics (IVD) Market Size By 2025?
The market size for cancer in vitro diagnostics (IVD) has seen significant growth in the past few years. The market value, which is expected to be $13.36 billion in 2024, is projected to increase to $14.32…
In Vitro Diagnostics (IVD) Market
With the watchful use of established and advanced tools such as SWOT analysis and Porter's Five Forces Analysis, this market report has been structured. While preparing this In Vitro Diagnostics (IVD) Market research report, few of the attributes that have been adopted include highest level of spirit, practical solutions, committed research and analysis, innovation, integrated approaches, and most up-to-date technology.
Every possible effort has been taken while researching and analysing…
Companion Animal IVD Market - Guiding the Path to Optimal Health: Empowering Vet …
Newark, New Castle, USA - new report, titled Companion Animal IVD Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Companion Animal IVD market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Companion Animal IVD market. The report offers an overview of…
IVD Market 2021 | Detailed Report
The IVD market research report delivers accurate data and innovative corporate analysis, helping organizations of all sizes make appropriate decisions. The IVD report also incorporates the current and future global market outlook in the emerging and developed markets. Moreover, the report also investigates regions/countries expected to witness the fastest growth rates during the forecast period.
The IVD research report also provides insights of different regions that are contributing market growth.…
Liquid Biopsy IVD Market 2021 | Detailed Report
According to Market Study Report, Liquid Biopsy IVD Market provides a comprehensive analysis of the Liquid Biopsy IVD Market segments, including their dynamics, size, growth, regulatory requirements, competitive landscape, and emerging opportunities of global industry. An exclusive data offered in this report is collected by research and industry experts team.
Get Free Sample PDF (including full TOC, Tables and Figures) of Liquid Biopsy IVD Market @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=4623688
The report provides a…
Asia IVD Market
According to a new report published by Allied Market Research, the Asia Pacific In-vitro diagnostics market was valued at $12.9 billion in 2015, and is expected to reach $19.0 billion registering a CAGR of 5.6% during 2016 to 2022. The report offers a detailed analysis of the key segments, top investment pockets, changing dynamics, market size & estimations, and competitive scenario.
Download Free Sample Report @ https://www.alliedmarketresearch.com/request-sample/1256
The Asia-Pacific IVD market is…
