Press release
U.S. Gastrointestinal Point of Care Testing (POCT) Market to Reach US$ 1.85 Bn by 2032 - Persistence Market Research
The U.S. gastrointestinal point of care testing (POCT) market is poised for significant growth over the coming years. Estimated at $926.0 million in 2025, the market is expected to nearly double by 2032, reaching $1.85 billion, with a compound annual growth rate (CAGR) of 10.4%. Several key factors are driving this expansion, including the rising prevalence of gastrointestinal (GI) disorders, the increasing demand for rapid and accessible diagnostic solutions, and technological advancements in diagnostic tools. GI disorders, such as infections, inflammatory bowel disease, and colorectal cancer, are among the most common and costly health conditions in the U.S. Consequently, the need for fast, reliable, and cost-effective diagnostics has spurred the adoption of point-of-care testing technologies, which allow healthcare providers to deliver timely treatment and make informed decisions at the point of care.Technological innovations, particularly in molecular diagnostics and immunoassays, have improved the accuracy, usability, and speed of POCT. These advancements are particularly significant for the early detection of GI diseases, which require swift intervention to prevent complications. Moreover, the growing emphasis on patient-centric, decentralized healthcare models has further accelerated the adoption of POCT. Decentralized healthcare, characterized by delivering care outside traditional clinical settings, aligns with the increasing demand for home-based and on-the-spot diagnostics. Government initiatives, such as those from the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA), are also fostering market growth by promoting preventive screenings and expanding access to point-of-care technologies, particularly for conditions like colorectal cancer.
Get a Sample Copy of Research Report (Use Corporate Mail id for Quick Response): https://www.persistencemarketresearch.com/samples/35231
Key Growth Drivers Behind Market Expansion
Several factors are driving the growth of the U.S. gastrointestinal point of care testing market. First, the rising burden of gastrointestinal diseases is a significant driver. According to the CDC, gastrointestinal infections are a leading cause of morbidity and healthcare utilization in the U.S., with over 179 million cases of acute gastroenteritis reported annually. The widespread nature of conditions such as norovirus, Clostridioides difficile, and Salmonella infections underpins the need for rapid diagnostic solutions. With an increasing number of people diagnosed with conditions like inflammatory bowel disease (IBD) and colorectal cancer, there is a growing demand for point-of-care diagnostic tools that can detect these diseases early.
Another key driver is the technological advancements in POCT. In recent years, innovations such as immunoassays and molecular diagnostics have improved the accuracy and speed of gastrointestinal tests. These technologies have significantly enhanced the ability of healthcare providers to diagnose and treat gastrointestinal conditions on-site, reducing patient wait times and improving overall healthcare efficiency. Additionally, the shift towards decentralized care has made POCT more critical, as patients and healthcare providers increasingly seek diagnostics that can be performed outside of traditional hospital settings.
Leading Segment and Geographical Region
Among the various segments of the gastrointestinal POCT market, assay kits dominate the market due to their high accuracy, broad application range, and fast turnaround times. These kits are used to detect a variety of gastrointestinal conditions, such as H. pylori, Clostridioides difficile, and Salmonella, making them essential tools in clinical settings like emergency departments, urgent care clinics, and even home-based healthcare. The growing prevalence of gastrointestinal infections and diseases has created a strong demand for these tools, further cementing their dominant position in the market.
Geographically, North America, particularly the United States, is the leading region in the gastrointestinal POCT market. This dominance is attributed to the high burden of gastrointestinal diseases, robust healthcare infrastructure, and the early adoption of advanced diagnostic technologies. The U.S. government has been proactive in promoting preventive healthcare, especially through the CDC and FDA's initiatives that focus on increasing access to POCT for colorectal cancer and other GI conditions. The growing trend towards home-based care and telemedicine in the U.S. is also expected to boost the demand for POCT solutions.
Key Highlights from the Report
• The U.S. gastrointestinal POCT market is projected to grow from $926.0 million in 2025 to $1.85 billion by 2032.
• Assay kits dominate the market, owing to their accuracy, broad application, and quick turnaround times.
• The demand for rapid diagnostic solutions is driven by the high prevalence of gastrointestinal infections and diseases.
• The adoption of decentralized healthcare is significantly increasing the demand for POCT technologies.
• The CDC and FDA are actively promoting preventive screening and expanding access to POCT, particularly for colorectal cancer.
• Technological advancements, such as molecular diagnostics and immunoassays, have improved the accuracy and speed of gastrointestinal POCT.
Market Segmentation
The U.S. gastrointestinal POCT market is segmented based on product type, end-user, and indication.
Product Type
In terms of product type, gastrointestinal POCT assay kits hold the largest market share, accounting for approximately 41.4% of the market in 2025. These kits, which include rapid immunoassays and molecular diagnostic tools, are used extensively across hospitals, clinics, and home settings. They offer high accuracy and a fast turnaround time, making them essential in managing gastrointestinal conditions. Dipsticks, primarily used for fecal occult blood testing (FOBT), represent the fastest-growing segment within the product category. Their simplicity, low cost, and ease of use make them particularly attractive for use in primary care and community health environments.
Additionally, cassette-based tests, often utilizing lateral flow assay technology, are gaining popularity. These tests are user-friendly, require minimal sample preparation, and are ideal for use in urgent care settings, physician offices, and telemedicine kits.
End-User Segmentation
The end-user segment of the gastrointestinal POCT market includes hospitals, clinics, diagnostic laboratories, and home care settings. Hospitals and clinics currently dominate the market due to the high volume of tests conducted in these environments. However, home care settings are emerging as an important segment, driven by the increasing popularity of at-home testing kits and telemedicine services. As more patients opt for home-based care, the demand for decentralized diagnostic solutions, such as at-home fecal immunochemical tests (FIT) for colorectal cancer, is expected to rise.
Indication Insights
In terms of indications, the primary driver of demand in the gastrointestinal POCT market is the need to diagnose infectious diseases rapidly. Bacterial infections, such as those caused by Clostridioides difficile, Salmonella, and Helicobacter pylori, contribute significantly to diagnostic demand. Additionally, viral infections like norovirus and rotavirus, which are widespread and highly contagious, create a need for quick, on-site testing, particularly in schools, long-term care facilities, and other communal environments. Colorectal cancer screening is also a growing area, with fecal immunochemical tests (FIT) becoming increasingly popular for early detection.
Read Detailed Analysis: https://www.persistencemarketresearch.com/market-research/us-gastrointestinal-point-of-care-testing-market.asp
Regional Insights
The U.S. holds the largest share of the gastrointestinal POCT market, driven by the high prevalence of gastrointestinal diseases and infections, as well as the advanced healthcare infrastructure. The government's efforts to promote preventive healthcare and improve access to diagnostic testing further bolster market growth.
In terms of regional trends, urban areas continue to drive demand for point-of-care diagnostic solutions due to their well-established healthcare systems and large patient populations. However, rural areas represent an underserved market, where the need for rapid, accessible diagnostic solutions is growing. The expanding availability of telemedicine services and mobile diagnostics is expected to drive growth in these regions, providing greater access to POCT for populations that traditionally lack timely access to healthcare.
Market Drivers
The primary driver of the U.S. gastrointestinal point of care testing market is the rising incidence of gastrointestinal diseases and infections. With over 179 million cases of acute gastroenteritis reported annually in the U.S., the need for fast, on-site diagnostic solutions is paramount. Conditions such as norovirus, Salmonella, and Helicobacter pylori are highly contagious and can lead to serious complications if not treated promptly. Point-of-care tests are crucial in reducing the time between symptom onset and treatment, thereby improving patient outcomes and preventing the spread of infectious diseases.
The growing shift toward decentralized healthcare is also a key driver. As patients increasingly seek care outside of traditional healthcare settings, the demand for easy-to-use and rapid diagnostic tools is growing. The convenience of home testing kits and telemedicine integration further supports this trend, providing patients with access to diagnostic results without having to visit a hospital or clinic.
Market Restraints
Despite the promising growth prospects, the U.S. gastrointestinal POCT market faces several challenges. One of the primary restraints is the accuracy concerns surrounding some rapid diagnostic tests. While POCT offers convenience and speed, certain tests may produce false positives or negatives, which could lead to misdiagnosis or delayed treatment. This is particularly concerning in critical care settings where diagnostic precision is essential.
Regulatory hurdles also act as a barrier to market expansion. The approval process for new diagnostic tools can be lengthy and complicated, which may delay the introduction of innovative products. Furthermore, reimbursement policies for POCT are not uniform across payers, which could limit the adoption of these technologies in some healthcare settings.
Market Opportunities
The U.S. gastrointestinal POCT market is ripe with opportunities, particularly in the development of innovative diagnostic technologies. Smartphone-compatible and digital POCT solutions are gaining traction, allowing patients to conduct tests at home and share results with healthcare providers in real-time. The popularity of telemedicine also offers a favorable environment for integrating POCT into remote care.
Additionally, there is a growing focus on preventive healthcare and early disease detection, which presents significant opportunities for POCT. Screening programs, especially for colorectal cancer, are driving demand for non-invasive diagnostic tools like fecal immunochemical tests (FIT). Rural and underserved areas also present an untapped market for POCT, as they often lack access to centralized laboratories.
Request for Customization of the Research Report: https://www.persistencemarketresearch.com/request-customization/35231
Company Insights
Several key players dominate the U.S. gastrointestinal POCT market:
• Abbott Laboratories
• QuidelOrtho Corporation
• Bio-Rad Laboratories
• Thermo Fisher Scientific
• BD (Becton, Dickinson and Company)
• Siemens Healthineers
• Prometheus Biosciences
• F. Hoffmann-La Roche Ltd
• Cardinal Health
• Luminex Corporation
• DiaSorin S.p.A.
Market Segmentation
By Product
Assay Kits
Dipsticks
Cassette
Others
By Test Type
Occult Blood Tests
H. pylori Test
Calprotectin Test
C. difficile Assays
Others
By Indication
Bacterial Infection
Viral Infection
Parasitic Infection
Colorectal Cancer
By Sample Type
Stool
Blood
Others
By End-user
Hospitals and Clinics
Diagnostic Laboratories
Ambulatory Surgical Centers
Urgent Care Centers
Home Care Settings
Others
Recent Market Developments
MP Biomedicals Launches New Diagnostic Kits (August 2024)
In August 2024, MP Biomedicals introduced a new line of in vitro diagnostic kits using immunochromatographic technology. These kits are designed to detect gastrointestinal pathogens such as Helicobacter pylori, Salmonella typhi, and Vibrio cholerae serogroups O1 and O139. This move further enhances the diagnostic options available for detecting gastrointestinal infections quickly and effectively.
Reese Pharmaceutical's ColoTest for At-Home Use (February 2024)
In February 2024, Reese Pharmaceutical launched ColoTest, an FDA-approved over-the-counter fecal immunochemical test (FIT) designed for at-home use. This test detects hidden blood in stool, which is a potential early warning sign of colorectal cancer and other gastrointestinal conditions. This innovation aligns with the growing trend toward decentralized healthcare and home-based diagnostics.
Conclusion
The U.S. gastrointestinal point of care testing (POCT) market is undergoing rapid transformation, driven by technological advancements, the increasing burden of gastrointestinal diseases, and the growing preference for decentralized healthcare. The market's growth is underpinned by the demand for faster, more accurate diagnostic tools that can be used in diverse settings-from hospitals and urgent care clinics to home-based care. Assay kits, particularly for detecting common GI pathogens and conditions like colorectal cancer, are expected to continue leading the market due to their broad applicability and fast results.
While the market faces challenges, including accuracy concerns and regulatory hurdles, opportunities in technological innovation and preventive care trends offer significant potential for further market expansion. Companies are actively investing in partnerships, acquisitions, and new product developments to cater to the increasing demand for accessible and reliable gastrointestinal diagnostics. With advancements in digital solutions, the U.S. gastrointestinal POCT market is positioned for sustained growth, benefiting both healthcare providers and patients seeking timely and efficient care.
The future of gastrointestinal POCT looks promising, with innovations that could not only improve diagnostic accuracy but also revolutionize the way healthcare is delivered. As awareness grows about the importance of early detection and the benefits of rapid diagnostics, the market is likely to see continued expansion, particularly in underserved and rural areas where access to traditional diagnostic tools may be limited. With growing government support and increasing consumer demand for home-based care, the U.S. gastrointestinal POCT market is set to play a crucial role in the future of healthcare in the country.
Read More Related Reports:
U.S. Triage System Market https://www.persistencemarketresearch.com/market-research/us-triage-system-market.asp
Africa Pharmaceuticals Market https://www.persistencemarketresearch.com/market-research/africa-pharmaceuticals-market.asp
Laboratory Management Services Market https://www.persistencemarketresearch.com/market-research/laboratory-management-services-market.asp
Burn Care Centers Market https://www.persistencemarketresearch.com/market-research/burn-care-centers-market.asp
Contact Us:
Persistence Market Research
Second Floor, 150 Fleet Street, London, EC4A 2DQ, United Kingdom
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release U.S. Gastrointestinal Point of Care Testing (POCT) Market to Reach US$ 1.85 Bn by 2032 - Persistence Market Research here
News-ID: 4247800 • Views: …
More Releases from Persistence Market Research
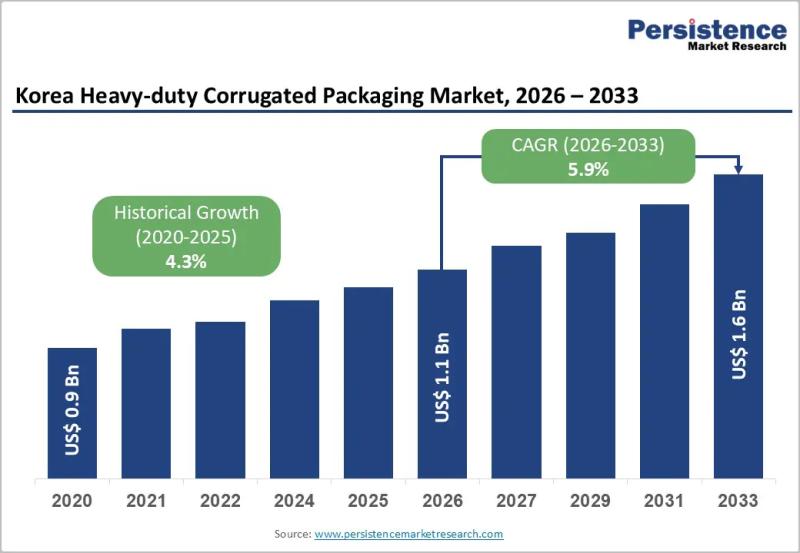
Korea Heavy-duty Corrugated Packaging Market to Reach US$1.6 Billion by 2033 - P …
The Korea heavy-duty corrugated packaging market plays a critical role in supporting industrial logistics, bulk transportation, and export-driven manufacturing. Heavy-duty corrugated packaging is widely used for shipping machinery, automotive components, electronics, chemicals, and large industrial goods that require superior strength and structural integrity. Unlike conventional corrugated boxes, heavy-duty variants are engineered with multi-wall boards, reinforced liners, and customized structural designs to withstand high load capacity, stacking pressure, and long-distance transportation.…

Textile Flooring Market Set for Steady Growth as Demand for Sustainable and Styl …
The global textile flooring market is entering a phase of stable expansion, supported by rising construction activity, increasing consumer focus on interior aesthetics, and growing demand for eco-friendly flooring solutions. According to industry estimates, the global textile flooring market size is likely to be valued at US$11.1 billion in 2026 and is projected to reach US$16.5 billion by 2033, expanding at a CAGR of 5.8% between 2026 and 2033. This…

Power System Simulator Market Size to Reach US$ 2.6 Billion by 2033 - Persistenc …
The power system simulator market is gaining strategic importance as global energy systems transition toward digitalization, decentralization, and decarbonization. Power system simulators are advanced software and hardware platforms used by utilities, grid operators, engineering firms, and research institutions to model, analyze, and optimize electrical power networks. These simulators enable real time grid analysis, contingency planning, load flow studies, fault analysis, stability assessment, and operator training. As electricity networks become more…

Yoga and Meditation Products Market Set for Robust Growth, Projected to Reach US …
The global wellness industry is undergoing a major transformation as consumers increasingly prioritize mental health, mindfulness, and preventive self-care. Within this evolving landscape, the yoga and meditation products market has emerged as a fast-growing segment, encompassing everything from yoga mats and apparel to meditation cushions, smart devices, and digital-enabled accessories. According to industry estimates, the global yoga meditation products market is projected to be valued at US$ 8.3 billion in…
More Releases for POCT
POCT Product and Devices Market Key Players, Share and Forecast Outlook
"The global Point-of-Care Testing (POCT) market is valued at approximately $35 billion in 2024, driven by technological advancements and a growing emphasis on decentralizing healthcare. By 2034, the market is projected to reach around $60 billion, demonstrating significant growth opportunities. This trajectory implies a robust Compound Annual Growth Rate (CAGR) of about 5.5% from 2025 to 2034."
Exactitude Consultancy., Ltd. released a research report titled "POCT Product and Devices Market". This…
Point-of-Care Testing (POCT) Market Size, Opportunities, Trends And Scope 2032
Point-of-Care Testing (POCT) Market Outlook & Investment Analysis
The Point-of-Care Testing (POCT) market is experiencing rapid growth due to increasing demand for rapid diagnostic solutions, technological advancements, and rising healthcare expenditure. The market is driven by the need for decentralized testing, particularly in remote and resource-limited areas. The growing prevalence of chronic diseases, such as diabetes and cardiovascular disorders, further accelerates adoption. North America dominates the market due to its well-established…
Molecular POCT Diagnostics Research:CAGR of 9.7% during the forecast period
Molecular POCT Diagnostics Market Summary
Molecular POCT (Point-of-Care Testing) Diagnostics refers to a diagnostic method that enables the detection of molecular markers-such as nucleic acids (DNA, RNA) or proteins-in biological samples (e.g., blood, saliva, or nasal swabs) directly at or near the site of patient care. Unlike traditional laboratory-based tests, which require sending samples to a central lab for analysis, molecular POCT diagnostics allow for rapid and decentralized testing, often providing…
India POCT Market Outlook to 2027F: Ken Research
India ranks at number 7 amongst the 20 wellness tourism markets with millions of trips made to India, medical value tourism generated multi-billion in revenue in 2022.
One of the nations where pricey medical procedures can be afforded is India. The cost of treatment in India is thought to be only 10% to 20% of what comparable procedures would cost in the West and other nations. India has a sizable number of hospitals with Joint Commission International…
Point-of-Care Testing (POCT) Market Current Scenario Forecast to 2027
The Global “Point-of-Care Testing (POCT)” Market Report 2021 to 2027 could be a fundamental examination of the global analysis. Trending innovation, advertise drivers, sectional declination, analysis measurements, advertise forecasts, manufacturers, and hardware merchants are all included within the substance. The report incorporates a point-by-point examination of the Point-of-Care Testing (POCT) Market as well as empowering advances, current patterns, openings, and obstruction, as well as a self-governing point of view, arrangement…
What Does POCT Mean?
POCT, which means point-of-care testing, is defined as medical diagnostic testing at or near the point of care. In these years, POCT received more and more attention, and we’d like to give a simple introduction in order to have a general idea about point-of-care.
What is POCT test?
POCT is medical abbreviation of Point-of-care testing, also known as bedside testing. It’s a form of testing which is designed to be used at…
