Press release
Pediatric Clinical Trials Market is expected to reach US$ 31.77 Billion by 2033 | Major key players - Pfizer Inc., ICON plc, Syneos Health.
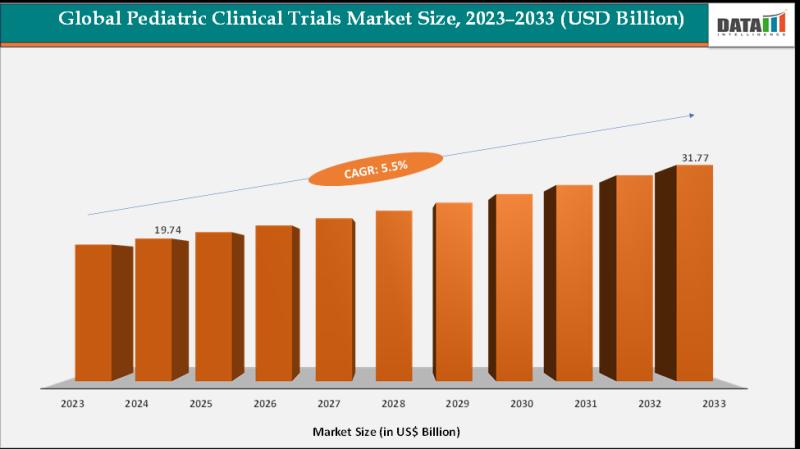
Market Size and Growth:The Global Pediatric Clinical Trials Market size reached US$ 19.74 Billion in 2024 from US$ 18.79 Billion in 2023 and is expected to reach US$ 31.77 Billion by 2033, growing at a CAGR of 5.5% during the forecast period 2025-2033. The Market growth is driven by increasing prevalence of pediatric diseases, rising demand for child-specific drug formulations, and supportive regulatory initiatives promoting pediatric research. According to DataM Intelligence
To Download a Free Sample PDF (Use Corporate email ID to Get Higher Priority) at: https://datamintelligence.com/download-sample/pediatric-clinical-trials-market?sz
The Pediatric Clinical Trials Market focuses on conducting research studies that evaluate the safety, efficacy, and dosing of drugs, vaccines, and medical devices in infants, children, and adolescents. It ensures age-appropriate treatments, ethical testing, and regulatory compliance to support pediatric healthcare advancements, drug approvals, and innovation in child-specific therapeutics globally.
United States: Industry Recent Developments
✅ August 2025: Labcorp launched a suite of central laboratory solutions called Labcorp Global Trial Connect to increase the speed and efficiency of clinical trials, particularly in areas like pediatric trials.
✅ September 2025: Bavarian Nordic commenced a Phase III clinical trial of its chikungunya vaccine CHIKV VLP targeting pediatric patients.
✅ October 2025: Increasing investment by government entities and private companies continues to drive innovation and expansion in pediatric therapies, supported by strong healthcare infrastructure and regulatory incentives in the US.
Japan: Industry Recent Developments
✅ August 2025: The PMDA emphasized new initiatives to promote pediatric drug development and clinical trials despite ongoing challenges in pediatric clinical studies.
✅ September 2025: In collaboration with pharmaceutical companies and academic institutions, the Japanese government expanded funding programs aimed at fostering pediatric drug development.
✅ October 2025: Clinical guidelines such as the ICH E11 continue to be the principal framework guiding pediatric clinical trials in Japan, supporting more comprehensive and detailed trial protocols.
Get Customization in the report as per your requirements + Exclusive Bundle & Multi-User Discounts: https://datamintelligence.com/customize/pediatric-clinical-trials-market?sz
FDA Approvals (2025):
✅ Cabometyx (cabozantinib) - expanded approval to include pediatric patients (≥12 years) - March 28, 2025 - FDA announced approval for adult and pediatric patients 12 years and older for certain neuroendocrine tumors.
✅ Moderna's Spikevax - BLA supplement approval with pediatric-related determinations - July 9, 2025 (approval letter) - FDA approved a supplement and documented pediatric assessment / PREA items in the approval letter. (This is relevant where COVID vaccine pediatric labeling/commitments affect pediatric immunization trials/policy).
✅ PenMENVY - approval letter (contains pediatric requirements / labeling references) - Feb 14, 2025 (approval letter PDF) - FDA approval letter references pediatric study/labeling obligations under PREA.
✅ BLA NDA reviews and new antiviral/biologic BLAs with pediatric review components (example: Enflonsia BLA integrated review completed 5 Jun 2025) - integrated FDA review documents show pediatric-drug development content and PDUFA dates in 2025. (useful for sponsors running pediatric trials).
✅ Note: FDA maintained multiple pediatric-related actions in 2025 (approvals, PREA obligations, pediatric exclusivity/grants and label expansions). FDA roundups and approval letters above are the primary sources for those items.
Mergers & Acquisitions (2025):
✅ Aveanna Healthcare acquires Thrive Skilled Pediatric Care - announced April 3, 2025 - $75M deal that expands home- and skilled-pediatric care capacity (affects site-of-care and post-trial care options).
✅ THL Partners (PE) definitive agreement to acquire Headlands Research - announced Aug 14, 2025 (deal reporting in Q3 2025) - Headlands is a multi-national clinical trial site network (including CNS, vaccines, metabolic diseases) - consolidation affects pediatric site networks and trial access/capacity.
✅ Notable pharma M&A with downstream pediatric impact - e.g., Merck's agreement to acquire SpringWorks (Apr 28, 2025) - large pharma buyouts reshuffle oncology portfolios (many pediatric oncology programs are affected by such consolidation).
✅ These transactions matter for pediatric trials because they change (1) site networks and investigator capacity, (2) sponsor portfolios and priorities for pediatric indications, and (3) CRO / CDMO dynamics that support pediatric-study supply chains.
Regulatory Approvals (2025):
✅ EMA: April 2025 update - mandatory use of EMA's IRIS portal for paediatric submissions - April 2025 guidance/industry updates - EMA required paediatric submissions to be made via IRIS, changing submission workflow for Paediatric Investigation Plans (PIPs) and related filings. This directly impacts sponsors running pediatric trials in the EU.
✅ FDA continued use of PREA/BPCA mechanisms; pediatric exclusivity listings updated in 2025 - FDA pages and pediatric-exclusivity lists were updated in 2025 showing ongoing pediatric study commitments and exclusivity grants (affects incentives and trial planning).
✅ EMA/CHMP 2025 meeting outputs and approvals - CHMP agendas and approvals in 2025 include paediatric-relevant decisions (affecting when sponsors must run pediatric studies or can get waivers). Track CHMP meeting notes for PIP decisions.
Major Key Players:
ICON plc
Pfizer Inc.
Syneos Health
Thermo Fisher Scientific Inc.
Medpace
Bristol-Myers Squibb Company
IQVIA Inc.
Parexel International Corporation
Charles River Laboratories.
Segments Covered in the Pediatric Clinical Trials Market:
By Phases: Phase I, Phase II, Phase III, Phase IV.
By Study Design: Treatment Studies, Observational Studies.
By Therapeutic Areas: Oncology, Autoimmune or Inflammatory Diseases, Respiratory Diseases, Infectious Diseases, Mental Health Disorders, Others.
Regional Analysis for Pediatric Clinical Trials Market:
⇥ North America (U.S., Canada, Mexico)
⇥ Europe (U.K., Italy, Germany, Russia, France, Spain, The Netherlands and Rest of Europe)
⇥ Asia-Pacific (India, Japan, China, South Korea, Australia, Indonesia Rest of Asia Pacific)
⇥ South America (Colombia, Brazil, Argentina, Rest of South America)
⇥ Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of Middle East & Africa)
Looking For Full Report? Get it Here: https://www.datamintelligence.com/buy-now-page?report=pediatric-clinical-trials-market
Chapter Outline:
⏩ Market Overview: It contains five chapters, as well as information about the research scope, major manufacturers covered, market segments, Pediatric Clinical Trials market segments, study objectives, and years considered.
⏩ Market Landscape: The competition in the Global Pediatric Clinical Trials Market is evaluated here in terms of value, turnover, revenues, and market share by organization, as well as market rate, competitive landscape, and recent developments, transaction, growth, sale, and market shares of top companies.
⏩ Companies Profiles: The global Pediatric Clinical Trials market's leading players are studied based on sales, main products, gross profit margin, revenue, price, and growth production.
⏩ Market Outlook by Region: The report goes through gross margin, sales, income, supply, market share, CAGR, and market size by region in this segment. North America, Europe, Asia Pacific, Middle East & Africa, and South America are among the regions and countries studied in depth in this study.
⏩ Market Segments: It contains the deep research study which interprets how different end-user/application/type segments contribute to the Pediatric Clinical Trials Market.
⏩ Market Forecast: Production Side: In this part of the report, the authors have focused on production and production value forecast, key producers forecast, and production and production value forecast by type.
⏩ Research Findings: This section of the report showcases the findings and analysis of the report.
⏩ Conclusion: This portion of the report is the last section of the report where the conclusion of the research study is provided.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Frequently asked questions:
➠ What is the global sales value, production value, consumption value, import and export of Pediatric Clinical Trials market?
➠ Who are the global key manufacturers of the Pediatric Clinical Trials Industry? How is their operating situation (capacity, production, sales, price, cost, gross, and revenue)?
➠ What are the Pediatric Clinical Trials market opportunities and threats faced by the vendors in the global Pediatric Clinical Trials Industry?
➠ Which application/end-user or product type may seek incremental growth prospects? What is the market share of each type and application?
➠ What focused approach and constraints are holding the Pediatric Clinical Trials market?
➠ What are the different sales, marketing, and distribution channels in the global industry?
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pediatric Clinical Trials Market is expected to reach US$ 31.77 Billion by 2033 | Major key players - Pfizer Inc., ICON plc, Syneos Health. here
News-ID: 4246727 • Views: …
More Releases from DataM Intelligence 4Market Research

Hydroponics Market is projected to reach USD 47.92 billion by 2032 | Major playe …
Market Size and Growth:
The Global Hydroponics Market size reached USD 16.54 billion in 2024 and is projected to reach USD 47.92 billion by 2032, growing at a CAGR of 14.22% during the forecast period 2025-2032.
The Hydroponics Market involves the cultivation of plants without soil, using nutrient-rich water solutions. It encompasses systems, equipment, and technologies for commercial and home-based hydroponic farming. Driven by urbanization, water efficiency, and demand for fresh produce,…

Hydrogen Compressor Market is expected to reach USD 2.8 billion by 2030 | Major …
Market Size and Growth:
The Global Hydrogen Compressor Market size reached USD 1.9 billion in 2022 and is expected to reach USD 2.8 billion by 2030, growing with a CAGR of 4.6% during the forecast period 2024-2031.
The Hydrogen Compressor Market involves the production, sales, and adoption of compressors designed to store, transport, and distribute hydrogen gas efficiently. These compressors are vital for fueling stations, industrial applications, and energy storage systems,…
Surgical Drapes Market is expected to reach US$ 2.74 billion by 2032 | Major pla …
Market Size and Growth:
The Global Surgical Drapes Market size reached US$ 1.44 billion in 2024 and is expected to reach US$ 2.74 billion by 2032, growing at a CAGR of 7.4% during the forecast period 2025-2032.
The Surgical Drapes Market encompasses the production, distribution, and use of sterile, disposable, or reusable drapes designed to maintain a sterile field during surgical procedures. These drapes protect patients from infection, prevent contamination, and enhance…

Gummy Vitamins Market is projected to reach USD 16.2 billion by 2031 | Major Com …
Market Size and Growth:
The Global Gummy Vitamins Market size reached USD 67.3 billion in 2022 and is projected to reach USD 16.2 billion by 2031, growing at a CAGR of 10.5% during the forecast period 2024-2031.
The Gummy Vitamins Market refers to the global industry involved in the production, distribution, and sale of chewable, flavored vitamin supplements designed for children and adults. These vitamins offer an alternative to traditional tablets or…
More Releases for Pediatric
Chronic Pediatric Conditions Fuels Growth In The Pediatric Radiology Market: An …
The Pediatric Radiology Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Projected Growth of the Pediatric Radiology Market?
The pediatric radiology market, valued at $5.94 billion in 2024, is projected to increase to $6.29 billion in 2025, reflecting a CAGR of 5.9%. Growth…
Pediatric Perfusion Market Experiences Growth with Increasing Pediatric Surgerie …
Market Overview
Pediatric Perfusion Market size was valued at US$ 575.3 Mn in 2025 and is expected to reach US$ 842.5 Mn by 2032 growing at a compound annual growth rate (CAGR) of 5.6% from 2025 to 2032.
Coherent Market Insights has released a comprehensive new report offering an in-depth analysis of the Pediatric Perfusion Market from 2025 to 2032. This study provides reliable global and regional projections, helping stakeholders navigate the…
Adorable Smiles Pediatric Dentistry: Pediatric Dentist Corinth, TX
Adorable Smiles Pediatric Dentistry [https://adorablesmilestx.com/] is proud to provide exceptional dental care for children in Corinth, TX. Conveniently located at 3901 FM 2181 Suite 140, the practice is a trusted choice for families looking for pediatric dental services in Corinth TX. With a fun, child-friendly atmosphere, the team ensures that every visit is enjoyable and stress-free for kids.
"Our goal is to create a positive dental experience for every child, building…
Pediatric Interventional Cardiology Market: Advancements and Opportunities for P …
The report is generically segmented into six parts and every part aims on the overview of the Pediatric Interventional Cardiology Market industry, present condition of the market, feasibleness of the investment along with several strategies and policies. Apart from the definition and classification, the report also discusses the analysis of import and export and describes a comparison of the market that is focused on the trends and development.
Along with…
Pediatric Neuroblastoma Treatment Market - Redefining Possibilities: Advancement …
Newark, New Castle, USA: The "Pediatric Neuroblastoma Treatment Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Pediatric Neuroblastoma Treatment Market: https://www.growthplusreports.com/report/pediatric-neuroblastoma-treatment-market/7681
This latest report researches the industry structure,…
Global Pediatric Vaccines Market | Global Pediatric Vaccines Industry | Global P …
The pediatric vaccines market involves of sales of pediatric vaccines and its linked services utilization to provide immunity to infants and children for specific syndromes. Pediatric vaccine is a preparation of killed the microorganisms, living tempered organisms, or living fully virulent organisms that are controlled to encourage the production of antibodies and deliver the immunity against one or numerous diseases among children. Pediatric vaccines are utilized in childhood immunization schedules…