Press release
United States AI in Clinical Trials Market 2025: Industry Developments, Future Growth, Share & Industry Insights | Medidata, IQVIA, Saama Technologies, Phesi, Euretos
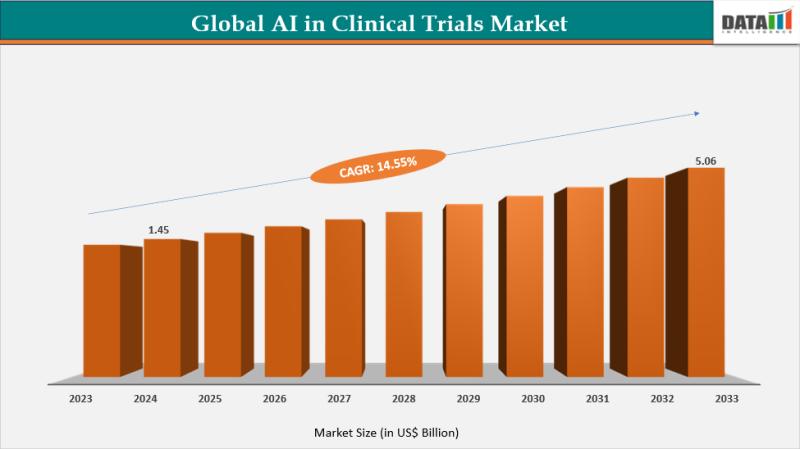
AI in Clinical Trials Market reached US$1.45 billion in 2024, up from US$1.27 billion in 2023, and is projected to reach US$5.06 billion by 2033, growing at a robust CAGR of 14.55% from 2025 to 2033.DataM Intelligence has published a new research report on "Al in Clinical Trials Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://datamintelligence.com/download-sample/ai-in-clinical-trials-market?sg
Key Players: Medidata, IQVIA, Saama Technologies, Phesi, Euretos, Median Technologies, Innoplexus, Deep6.ai, AiCure, Antidote Technologies and Mendel AI.
USA - Industry Latest News:
✅ 29 Oct 2025 - Thermo Fisher to acquire Clario (all-cash deal up to $9.4B). Clario is a clinical-trial software/data company whose platform is widely used to manage trials and generate trial data insights; Thermo Fisher says the deal will deepen its AI and digital capabilities across clinical research.
✅ 02 Jun 2025 - FDA launches agency-wide generative AI tool "Elsa" to speed internal scientific review and operations. Agency says the tool is being used to reduce repetitive tasks in regulatory review workflows.
✅ 08 May 2025 - FDA completes first AI-assisted scientific review pilot. Agency reported faster review cycles using generative AI tools during pilot work.
✅ 22 Aug 2025 - Tempus announces acquisition of Paige (AI pathology / cancer-analytics company). Deal expands Tempus' AI capabilities for clinical and translational oncology data used in trials.
✅ 14 Aug 2025 - Eli Lilly enters deal with Superluminal (AI-driven discovery) to develop obesity candidates; platforms expected to feed programmes that will move into human trials.
✅ 14 Aug 2025 - THL Partners agreement to acquire Headlands (U.S. clinical site network) from KKR - investor activity reflects demand for trial operations boosted by digital/AI tools.
Japan - Industry Latest News:
✅ 16 Oct 2025 - A.D.A.M. Innovations partners with SOPHiA GENETICS to adopt SOPHiA's AI-powered genomics platform for liquid biopsy & companion diagnostics in Japan. Partnership aims to accelerate precision-oncology testing that can be used in clinical trials and diagnostics.
✅ 25 Sep 2025 - Nakanoshima / Osaka initiatives accelerate industry-academia-government ecosystem to attract trials and AI-driven drug development. (Regional program and cluster activity to increase clinical trial inflow and data ecosystem readiness.)
✅ (2025) - Fujitsu launches global initiative to attract clinical trials to Japan and build medical-data ecosystem using advanced AI and trial platforms (collaboration with global partners to connect data and accelerate trial readiness).
South Korea (Korea) - Industry Latest News:
✅ 27 Oct 2025 - Kolmar Korea selected for government-led "AI Factory" initiative (Bio Division) to build integrated data platforms and AI models part of broader national AI push that will support clinical R&D data infrastructure.
✅ 03 Sep 2025 - Korea announces multi-million medical AI projects (e.g., My Healthway) connecting hospitals and data sources to support clinical research and AI-enabled health services. These national projects strengthen data pipelines for future AI-enabled clinical trials.
Europe - Industry Latest News:
✅ 21 Oct 2025 - European Commission launches flagship "Apply AI" initiative to speed up trustworthy AI in healthcare (including diagnostics and drug development support tools). The initiative is intended to accelerate adoption of EU-compliant AI in healthcare and related clinical workflows.
✅ 15 Oct 2025 - EssilorLuxottica acquires RetinAI to accelerate AI and data-powered eye-health solutions (example of health-AI consolidation in Europe with downstream clinical implications).
✅ Oct 2025 - Multiple strategic AI + biotech partnerships and deals reported across Europe (see deal roundups highlighting partnerships using AI for trial design, patient selection and imaging analytics).
Growth Forecast Projected:
The Global Al in Clinical Trials Market is anticipated to rise at a considerable rate during the forecast period, between 2025 and 2032. In 2024, the market is growing at a steady rate, and with the rising adoption of strategies by key players, the market is expected to rise over the projected horizon.
Research Process:
Both primary and secondary data sources have been used in the global Al in Clinical Trials Market research report. During the research process, a wide range of industry-affecting factors are examined, including governmental regulations, market conditions, competitive levels, historical data, market situation, technological advancements, upcoming developments, in related businesses, as well as market volatility, prospects, potential barriers, and challenges.
Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=ai-in-clinical-trials-market?sg
Key Segments:
By Offering (Software and Services)
By Trial Phase (Phase I, Phase II, and Phase III)
By Indication (Oncology, Central Nervous System (CNS) Disorders, Cardiovascular (CVS) Diseases, and Other Indications),
By End-user (Pharmaceutical and Biotechnology Companies, Contract Research Organizations (CROs), Other End Users)
By Region (North America, Europe, Asia-Pacific, South America, and the Middle East & Africa)
Regional Analysis for Market:
⇥ North America (U.S., Canada, Mexico)
⇥ Europe (U.K., Italy, Germany, Russia, France, Spain, The Netherlands and Rest of Europe)
⇥ Asia-Pacific (India, Japan, China, South Korea, Australia, Indonesia Rest of Asia Pacific)
⇥ South America (Colombia, Brazil, Argentina, Rest of South America)
⇥ Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of Middle East & Africa)
Benefits of the Report:
Chapter 1: Sets the stage by outlining the report's coverage, summarizing key market segments by region, product type, and application. Presents a snapshot of market sizes, growth potential across segments, and anticipated industry evolution both short and long term.
Chapter 2: Highlights pivotal market insights and uncovers the most significant emerging trends driving change within the industry.
Chapter 3: Offers an in-depth look at the competitive landscape among Al in Clinical Trials producers, including revenue shares, strategic moves, and recent mergers and acquisitions.
Chapter 4: Presents comprehensive profiles of the market's key players, delving into details such as revenue, profit margins, product portfolios, and company milestones.
Chapters 5 & 6: Analyze Al in Clinical Trials revenue at both regional and country levels, providing quantitative breakdowns of market sizes, growth opportunities, and development prospects worldwide.
Chapter 7: Focuses on different market segments by type, examining their individual sizes and potential, guiding readers toward high-impact, untapped market areas.
Chapter 8: Explores segmentation by application, evaluating industry growth potential in various downstream markets and pinpointing promising sectors for expansion.
Chapter 9: Provides a thorough review of the industry's supply chain mapping out both upstream and downstream activities.
Chapter 10: Concludes with a summary of the report's key findings and highlights the most critical takeaways for industry stakeholders.
Get Customization in the report as per your requirements: https://datamintelligence.com/customize/ai-in-clinical-trials-market?sg
FAQ's
Q1: What is the current size of the Al in Clinical Trials Market?
A: The Al in Clinical Trials Market stood at US$1.45 billion in 2024 and is set to experience remarkable growth, reaching US$5.06 billion by 2033
Q2: How fast is the Al in Clinical Trials Market growing?
A: The Market is on an impressive growth trajectory, expected to expand at a CAGR of 14.55% from 2025 to 2033.
Have any Enquiry of This Report @ https://www.datamintelligence.com/enquiry/ai-in-clinical-trials-market?sg
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release United States AI in Clinical Trials Market 2025: Industry Developments, Future Growth, Share & Industry Insights | Medidata, IQVIA, Saama Technologies, Phesi, Euretos here
News-ID: 4246690 • Views: …
More Releases from DataM Intelligence 4 Market Research LLP
United States Induction Motor Market 2031 | Growth Drivers, Trends & Market Fore …
Induction Motor Market size was worth US$ 20.36 billion in 2023 and is estimated to reach US$ 33.66 billion by 2031, growing at a CAGR of 6.49% during the forecast period (2024-2031).
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/induction-motor-market?kb
List of Top Key Player:
ABB Ltd., Ametek, Emerson Electric, Siemens AG, Brook Crompton, Danaher Corporation, Johnson Electric Holdings, Regal Beloit, WEG Electric Corp.…
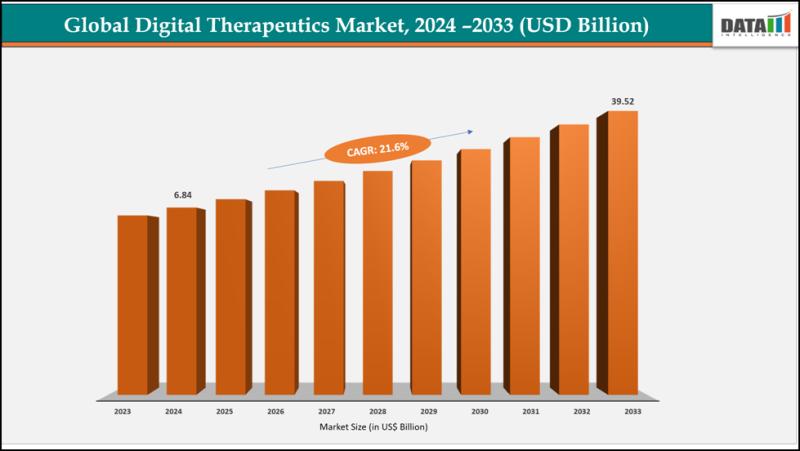
Digital Therapeutics Market Set for Explosive Growth to USD 39.52 Billion by 203 …
The Global Digital Therapeutics Market size reached USD 6.84 billion in 2024 and is expected to reach USD 39.52 billion by 2033, growing at a CAGR of 21.6% during the forecast period 2025-2033.
Market growth is driven by the rising prevalence of chronic diseases like diabetes and mental health disorders, increasing smartphone penetration, and growing patient demand for personalized, app-based interventions. Advancements in AI and machine learning for behavior change, expanding…
United States Contrast Media Injectors Market: Real-Time Market Trends & Competi …
DataM Intelligence unveils its latest report on the "Contrast Media Injectors Market Size 2025," offering an in-depth analysis of market trends, growth drivers, competitive landscape, and regional dynamics. The study covers market size in value and volume, CAGR forecasts, and emerging opportunities that can guide businesses in seizing growth potential and crafting winning strategies. Packed with data-driven insights on current developments and future trends, this report is essential for companies…

United States Real Time Location System (RTLS) Market Analysis 2026: Growth Driv …
Real Time Location System (RTLS) Market is expected to grow at a CAGR of 18% during the forecasting period (2022-2029).
Request a Premium Sample PDF of This Report (Corporate Email IDs Receive Priority Service): https://www.datamintelligence.com/download-sample/real-time-location-system-market?kb
United States: Recent Industry Developments
✅ December 2025: Major healthcare systems expanded RTLS deployments to enhance patient tracking, asset utilization, and workflow efficiency.
✅ November 2025: Leading tech providers integrated AI‐driven analytics into RTLS platforms to deliver predictive…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Management System
According to a new market report published by Persistence Market Research “Global Market Study on Clinical Trial Management System: Asia to Witness Highest Growth by 2019” the global clinical trial management system market was valued at USD 844.0 million in 2013 and is expected to grow at a CAGR of 14% from 2014 to 2019, to reach an estimated value of USD 1,848.5 million in 2019.
Request Report TOC @ https://www.persistencemarketresearch.com/methodology/3017
…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…