Press release
Decentralized Clinical Trials Market Set for Explosive Growth, Hitting USD 46 Billion by 2035
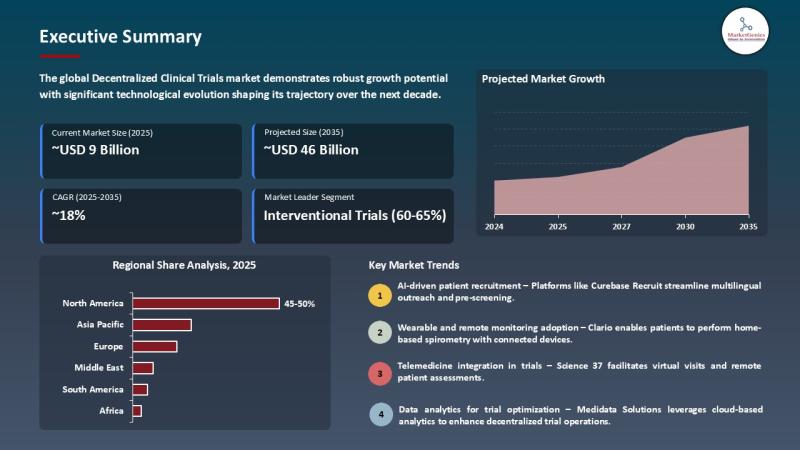
The global decentralized clinical trials market is projected to expand from USD 9.1 billion in 2025 to USD ~46 billion by 2035, registering a CAGR of 17.6% over the forecast period. The global decentralized clinical trials (DCT) market is reshaping the world of clinical research at a very fast rate by transforming conventional, site-based trials to remote and patient-based ones. Using digital health technologies, telemedicine, wearables, and eClinical platforms, DCTs allow collecting data in real-time, monitoring patients better, and recruit more participants regardless of the geography.The method increases patient convenience, minimizes the dropouts, and shortens the study timelines and decreases operational expenses. The main drivers in the market are the growing use of remote monitoring solutions, the use of AI to recruit patients, and the need to develop drugs faster and at a lower cost. The market is characterized by strong control of North America because of a strong infrastructure in healthcare, high technology adoption, and a conducive regulatory environment, and Europe and Asia-Pacific are experiencing an increasing trend. There are still challenges of data privacy, regulatory barriers, and integration of technology, but the continuous growth of the market is driven by new innovations and digital health investments.
Get the Detailed Industry Analysis (including the Table of Contents, List of Figures, and List of Tables) - from the Decentralized Clinical Trials Market Research Report: https://marketgenics.co/press-releases/decentralized-clinical-trials-market-80879
Key Driver, Restraint, and Growth Opportunity Shaping the Global Decentralized Clinical Trials Market
The increasing need of real-world evidence (RWE) is accelerating the use of decentralized clinical trials (DCTs) as remote data collection in the daily surroundings of patients offers a more detailed, ongoing, and precise contribution to drug development and post-market research. As an example, IQVIA uses real-world data (RWD) to create evidence (RWE), which allows insights about the performance of treatments and their costs and patient outcomes, supporting decentralized trials and creating comprehensive analyses that are not possible in a clinical environment.
Global decentralized clinical trials have major challenges in the absence of standardized regulations in the different regions and this makes it hard to achieve uniform data privacy of patients, secure digital data management, receive multi-country approvals, and adhere to various ethical and legal standards.
The increasing use of hybrid trials is a great opportunity, as a combination of site visits and remote monitoring can enable sponsors to increase patient retention and lower operational expenses, and make trials more accessible worldwide. For instance, Parexel conducted hybrid trials, which involved the use of electronic medical record (EMR) data alongside site-based collection on a trial on metastatic breast cancer, to increase the number of patients involved and allow more in-depth evaluation of real-world data.
To know more about the Decentralized Clinical Trials Market - Download our Sample Report: https://marketgenics.co/download-report-sample/decentralized-clinical-trials-market-80879
Regional Analysis of Global Decentralized Clinical Trials Market
North America is the most decentralized clinical trial market due to its advanced healthcare system, centralization of pharmaceutical and biotech companies, and advanced research centers are working on or actively using decentralized and hybrid trial models. The presence of a variety of clinical trial locations, digital health technology delivery, and CRO activity in the area will allow the rapid recruiting of patients, remote patient monitoring, and the competent collection of data. For an instance, Science 37 and Catalent developed a strategic alliance in September 2025, so it would be able to enable a higher level of decentralized clinical trials by means of diversified patient-centric logistics and sophisticated trial operations.
The Asia Pacific area is a region with the fastest growth rate concerning the decentralized clinical trials (DCT) because the digital health technologies, telemedicine, and remote patient monitoring solutions are being introduced at an incredible pace. The expansion of DCT is also being driven by government-driven healthcare infrastructure and expansion in clinical trial programs and generally the patient convenience and access. The area is characterized by a high number of treatment-naive patients and the increased research and development of pharmaceutical and biotech industries.
Prominent players operating in the global decentralized clinical trials market are Clario (formerly ERT), Clinical Ink, Datacubed Health, Florence Healthcare, ICON plc, IQVIA Holdings Inc., LEO Innovation Lab, Medable Inc., Medidata Solutions (Dassault Systèmes), Medpace Holdings, Inc., Medrio, ObvioHealth, Oracle Corporation (Oracle Health Sciences), Parexel International Corporation, PRA Health Sciences (now part of ICON), Science 37 Holdings, Inc., Signant Health, Syneos Health, Thread, WCG (WIRB-Copernicus Group), YPrime, Other Key Players.
Buy Now: https://marketgenics.co/buy/decentralized-clinical-trials-market-80879
North America Leads Global Decentralized Clinical Trials Market Demand
North America leads the global market in the decentralized clinical trials market, with a market share of approximately 48%. The region's leadership is driven by well-established healthcare infrastructure, high adoption of digital health technologies, strong regulatory support, and the presence of major pharmaceutical and biotechnology companies. Increasing patient demand for convenient, remote participation in clinical studies, coupled with rising awareness of decentralized trial benefits, further fuels decentralized clinical trials market growth.
Additionally, favorable reimbursement policies, advanced telemedicine networks, and widespread use of wearable devices and ePRO/eCOA platforms make North America a hub for patient-centric clinical research. For instance, ObvioHealth, a U.S.-based Virtual Research Organization, conducts fully virtual decentralized trials using its ObvioGo platform, which integrates eConsent, ePRO, symptom tracking, and two-way communication to enable remote patient participation.
The strong research infrastructure and investments based on innovations in North America make it as the dominant center.
Get a preview of our Decentralized Clinical Trials Market Playbook - your guide to GTM strategy, competitive intelligence, supplier dynamics, and Consumer Behavior Analysis: https://marketgenics.co/playbook/decentralized-clinical-trials-market-80879
Key Trend: AI & Predictive Analytics and Cloud-Based Platforms enhancing trial efficiency
The Artificial Intelligence (AI) and predictive analytics is transforming the decentralized form of clinical trial, making it easier to recruit patients, optimize the trial design and monitor it in real-time. Increased use of AI-based applications can improve trial results and minimize expenses by analyzing large numbers of data to help identify the appropriate participants, forecast possible riskiness, and tailor the treatment plan. To provide an example, the Recruit of Curebase is an AI-based service that simplifies the recruitment of patients to decentralized trials by using multilingual outreach, pre-screening, referrals and ROI tracking.
The use of cloud-based platforms is central to the decentralized clinical trial management through scalable, flexible, and secure data solutions. These platforms allow sharing of real-time data between various sites, enhance collaboration, and decision-making. For an instance, the Cloud API of Empatica is used to combine real-time wearable information with Clinical Trial Management Systems (CTMS) and Electronic Data Capture (EDC) systems to enhance the accuracy of data and the efficiency of the trials.
As a result, AI-based technologies and cloud-based solutions are supporting decentralized clinical trials by enhancing patient recruitment, real-time monitoring, and general trial efficiency.
Contact:
Mr. Debashish Roy
MarketGenics India Pvt. Ltd.
800 N King Street, Suite 304 #4208, Wilmington, DE 19801, United States
USA: +1 (302) 303-2617
Email: sales@marketgenics.co
Website: https://marketgenics.co
About Us
MarketGenics is a global market research and management consulting company empowering decision makers across healthcare, technology, and policy domains. Our mission is to deliver granular market intelligence combined with strategic foresight to accelerate sustainable growth.
We support clients across strategy development, product innovation, healthcare infrastructure, and digital transformation.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Decentralized Clinical Trials Market Set for Explosive Growth, Hitting USD 46 Billion by 2035 here
News-ID: 4246655 • Views: …
More Releases from MarketGenics India Pvt. Ltd.
APAC Deepfake Detection Market Accelerates as Governments Tighten Digital Trust …
A Market Transforming How the World Verifies Reality
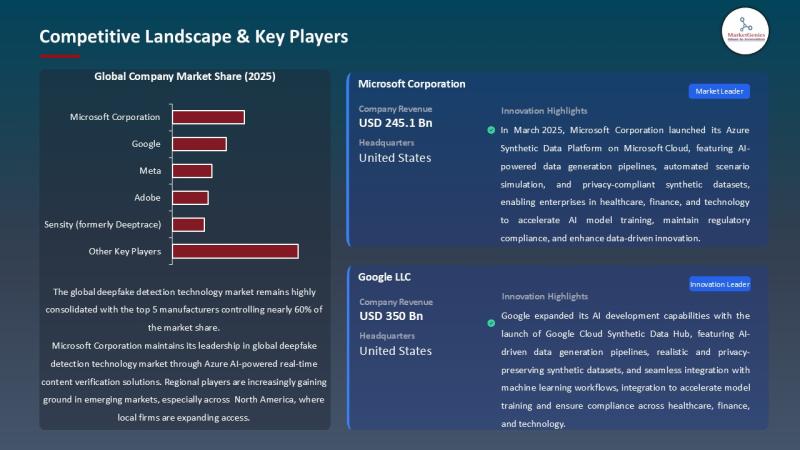
The global deepfake detection technology market, valued at USD 0.6 billion in 2025, is positioned to accelerate at a powerful 37.2% CAGR, reaching USD 15.1 billion by 2035.
This growth is driven by one undeniable truth:
Synthetic media is reshaping the threat landscape faster than humans can recognize it.
Deepfake detection technologies now determine:
How newsrooms verify breaking content
How financial institutions prevent identity-spoofing
How governments protect election integrity
How…
Travel & Expense Management Software Market Signals a Digital Pivot | AI, Cloud …
The Travel and Expense Management (TEM) Market Crossroads | A Sector Accelerating, Repricing Efficiency, and Redrawing the Corporate Spend Map
(Is TEM a Back-Office Tool-or the Operating System of the Next Enterprise Economy?)
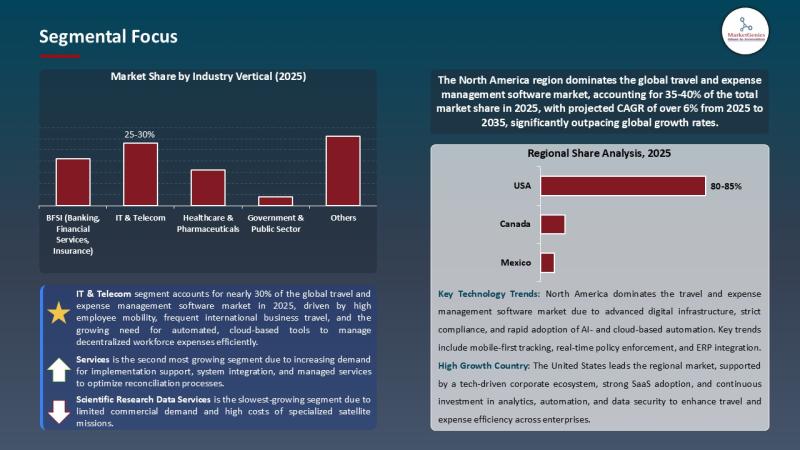
For years, the travel and expense management software market lived in the administrative shadows-handed off to finance teams, constrained by spreadsheets, and dismissed as a routine cost-control tool. But the numbers now tell a radically different story.
In 2025, the…
Oilfield Equipment Market hits USD 116.2B in 2025 and grows to USD 156.5B by 203 …
Oilfield Equipment Market | The $156.5B Hardware Backbone of the Global Energy System
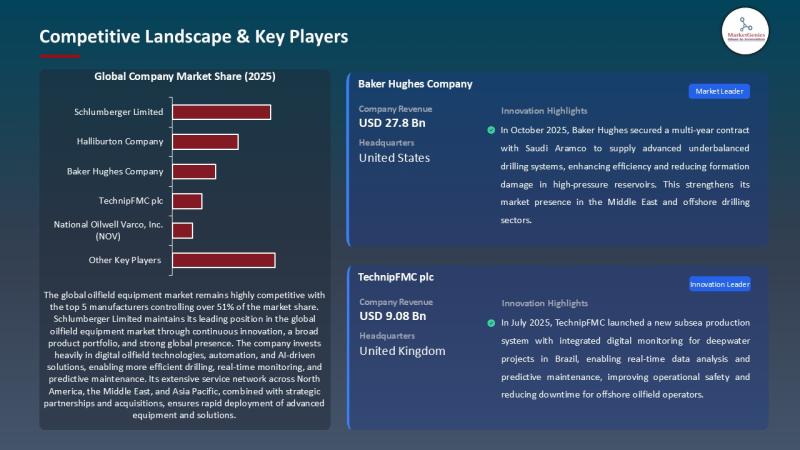
Every headline loves clean energy. Yet the global energy mix still demands a brutal truth: oil and gas remain the world's primary supply of heat, mobility, and petrochemicals - and the machines that drill, lift, complete, and produce hydrocarbons continue to define industrial capability.
That's why the Oilfield Equipment Market remains a strategic industry - not a relic.
In 2025,…

Machine Tools Market 2025-2035 | USD 109.9B Growth, CNC & Automation Trends
Machine Tools Market | The $109.9B Intelligence Engine of Global Manufacturing
Factories don't work without machine tools. They shape, cut, drill, grind, and define the physical world around us. Yet most end-products - cars, aircraft parts, electronics housings, surgical devices - never reveal the precision machinery behind them.
The Machine Tools Market is the invisible infrastructure that turns digital models into physical reality.
In 2025, the global Machine Tools Market stands at USD…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…