Press release
Clinical Translation Market Set to Reach USD 3.4 Billion by 2035 Amid Rising R&D Investments
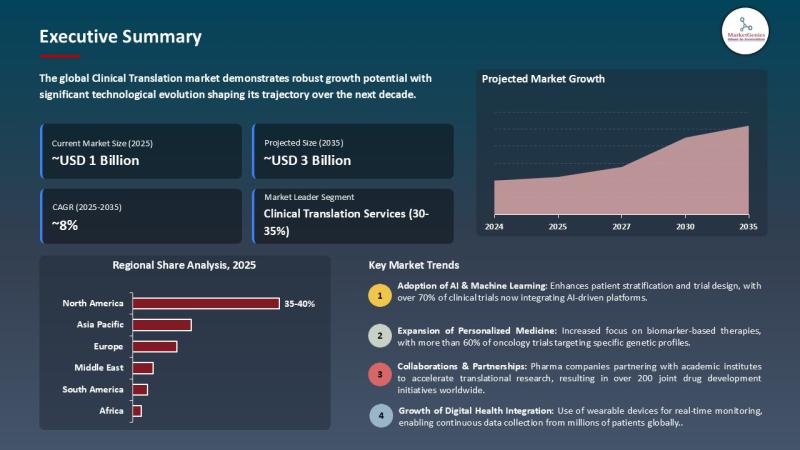
According to the MarketGenics report, the global clinical translation market is projected to expand from USD 1.6 billion in 2025 to USD 3.4 billion by 2035, registering a notable CAGR of 7.8% during the forecast period. The market of clinical translation revolves around the services and technologies that can be used to bridge the gap between laboratory research and clinical use so that discoveries of preclinical use could be safely and efficiently used in clinical trials and therapeutic intervention in humans. It comprises of activities like translation of clinical protocols, translation of regulatory documents, translation of patient recruitment materials, and multilingual data management which makes meaning in international clinical trials to be accurate, compliant, and understandable.The market motivates increasing chronic and rare disease rates, global clinical trial growth necessitating cross-cultural interaction, the development of precision medicine, biologics, and digital health technologies, and the strict regulatory frameworks with the focus on the standardized documentation and compliance. The major stakeholders are embracing AI-supported translators and decentralized trial technologies to simplify clinical activities across the globe. These factors will enable the clinical translation market to grow steadily, as the world continues to become globalized in clinical research practices and will continue to demand accurate and culturally tailored communication with a diverse range of patients.
Get the Detailed Industry Analysis (including the Table of Contents, List of Figures, and List of Tables) - from the Clinical Translation Market Research Report: https://marketgenics.co/press-releases/clinical-translation-market-30644
Key Driver, Restraint, and Growth Opportunity Shaping the Global Clinical Translation Market
The increasing globalization of clinical trials is a key force behind the clinical translation market, necessitating proper, multilingual and culturally translated protocols, informed consent forms, and patient documentation to meet regulatory requirements and ensure successful interaction with patients in different regions. For instance, Parexel has been expanding its presence in India with the firm intending to hire more than 2,000 employees in the next three to five years. With an investment of this magnitude, Parexel is currently carrying out 100 to 150 trial sites within the several states of India, which is reflecting the emerging trend as a place where early phase clinical trials are carried out.
The major limitation of the clinical translation market is the risk of data privacy and security breaches. In many cases, clinical translation deals with the sensitive information about patients, such as medical history, genetic data, and trial outcomes. The unauthorized access, data leak, or other cyberattack, which occur during the translation process, may harm patient confidentiality, break the laws and regulations, such as the HIPAA or GDPR, and result in legal and financial effects concerning the sponsors and translation providers.
The transition to decentralized and virtual clinical trials presents an opportunity where the clinical translation market offers the correct, multilingual documentation and better patient interaction in the regions across the globe. As an example, IQVIA, a major global CRO, provides decentralized clinical trial (DCT) services in more than 75 countries and 30 therapeutic areas, and has more than 500 active DCTs. Its patient-centered model that incorporates telehealth, mobile nursing, and distance monitoring fosters efficiency and effectiveness of clinical trials throughout the world and sustains the growing clinical translation market.
To know more about the Clinical Translation Market - Download our Sample Report: https://marketgenics.co/download-report-sample/clinical-translation-market-30644
Regional Analysis of Global Clinical Translation Market
The clinical translation market in North America displays the greatest demand because most of the research institutions, pharmaceutical firms as well as medical facilities are concentrated in the region and they actively participate in international clinical trials. Companies like IQVIA, ICON plc, and Labcorp Drug Development use the superior services of clinical translation to handle language-based regulatory submissions, patient-related documents, and protocol documentation in various geographical locations. Also, the availability of big contract research organizations (CROs) and sponsors guarantees the popularity of new translation technologies, AI-based monitoring, and decentralized trial solutions, which makes North America a place of state-of-the-art clinical translation projects in academic and industry research.
The fastest growth is projected in the clinical translation market of Asia Pacific due to the rapid growth in the scientific facilities, the rise in pharmaceutical research and development and the rise in investments in the advanced technologies in the medical care. Other countries like China, Japan, and India are strengthening clinical research and translation capabilities, driving growth in the global clinical translation market. For instance, Tridindia, the Indian language services provider, provided clinical trials with excellent localized translation of more than 200 languages. This reinforced the position of India in the developing Asia Pacific clinical translation market.
Prominent players operating in the global clinical translation market are Accelerated Enrollment Solutions, Altasciences, Charles River Laboratories, CluePoints, ICON plc, inVentiv Health (now Syneos Health), IQVIA (formerly Quintiles IMS), Labcorp Drug Development (formerly Covance), Medpace, Novotech, Oracle Health Sciences, Parexel International, PPD (Part of Thermo Fisher Scientific), PRA Health Sciences (Part of ICON), Premier Research, ProMedica Clinical Research, PSI CRO, SGS Clinical Research, Syneos Health, Theorem Clinical Research, Transperfect Life Sciences, Veristat, Worldwide Clinical Trials (Part of Fortrea), WuXi AppTec, and Other Key Players.
Buy Now: https://marketgenics.co/buy/clinical-translation-market-30644
Recent Development and Strategic Overview:
In October 2025, AstraZeneca's Baxdrostat completed a late-stage trial, significantly lowering blood pressure in patients with resistant hypertension. The drug, acquired via the $1.8 billion CinCor purchase in 2023, targets aldosterone to reduce cardiovascular risk.
In September 2025, Parexel announced a collaboration with Weave Bio to accelerate regulatory submission processes using AI. By leveraging Weave's AutoIND platform, Parexel has been able to complete Investigational New Drug (IND) applications 50% faster than traditional timelines, streamlining the initiation of clinical trials.
Get a preview of our Clinical Translation Market Playbook - your guide to GTM strategy, competitive intelligence, supplier dynamics, and Consumer Behavior Analysis: https://marketgenics.co/playbook/clinical-translation-market-30644
Key Trend: Integration of Digital Health and AI Accelerates Clinical Translation Efficiency
The clinical translation market is increasingly leveraging digital health technologies and artificial intelligence (AI) to enhance the efficiency and accuracy of drug development.
AI-driven platforms enable rapid drug discovery by analyzing vast datasets, identifying potential targets, and predicting efficacy and safety profiles before clinical testing. Predictive modeling helps optimize trial design, patient selection, and dosing strategies, reducing the risk of trial failures and shortening development timelines. For instance, in 2025, Insilico Medicine used its AI platform Pharma.AI to identify targets, design molecules, and predict clinical outcomes, advancing its AI‐discovered drug rentosertib into Phase IIa trials for idiopathic pulmonary fibrosis.
Moreover, digital patient monitoring and wearable technologies provide real-time data on treatment responses, adherence, and adverse events, improving trial outcomes and patient-centric decision-making. For instance, Nutromics, a med-tech company developed a wearable "lab-on-a-patch" that continuously monitors multiple biomarkers to detect conditions like sepsis and heart attacks and guide treatment decisions in real time.
Contact:
Mr. Debashish Roy
MarketGenics India Pvt. Ltd.
800 N King Street, Suite 304 #4208, Wilmington, DE 19801, United States
USA: +1 (302) 303-2617
Email: sales@marketgenics.co
Website: https://marketgenics.co
About Us
MarketGenics is a global market research and management consulting company empowering decision makers across healthcare, technology, and policy domains. Our mission is to deliver granular market intelligence combined with strategic foresight to accelerate sustainable growth.
We support clients across strategy development, product innovation, healthcare infrastructure, and digital transformation.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Translation Market Set to Reach USD 3.4 Billion by 2035 Amid Rising R&D Investments here
News-ID: 4246642 • Views: …
More Releases from MarketGenics India Pvt. Ltd.
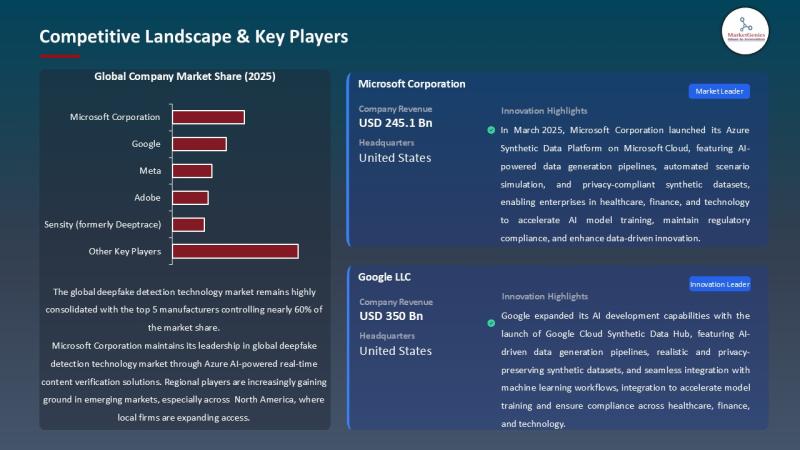
APAC Deepfake Detection Market Accelerates as Governments Tighten Digital Trust …
A Market Transforming How the World Verifies Reality
The global deepfake detection technology market, valued at USD 0.6 billion in 2025, is positioned to accelerate at a powerful 37.2% CAGR, reaching USD 15.1 billion by 2035.
This growth is driven by one undeniable truth:
Synthetic media is reshaping the threat landscape faster than humans can recognize it.
Deepfake detection technologies now determine:
How newsrooms verify breaking content
How financial institutions prevent identity-spoofing
How governments protect election integrity
How…
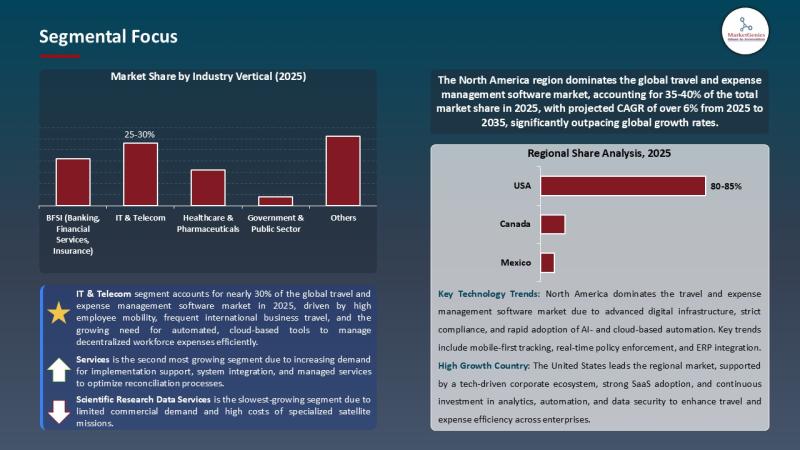
Travel & Expense Management Software Market Signals a Digital Pivot | AI, Cloud …
The Travel and Expense Management (TEM) Market Crossroads | A Sector Accelerating, Repricing Efficiency, and Redrawing the Corporate Spend Map
(Is TEM a Back-Office Tool-or the Operating System of the Next Enterprise Economy?)
For years, the travel and expense management software market lived in the administrative shadows-handed off to finance teams, constrained by spreadsheets, and dismissed as a routine cost-control tool. But the numbers now tell a radically different story.
In 2025, the…
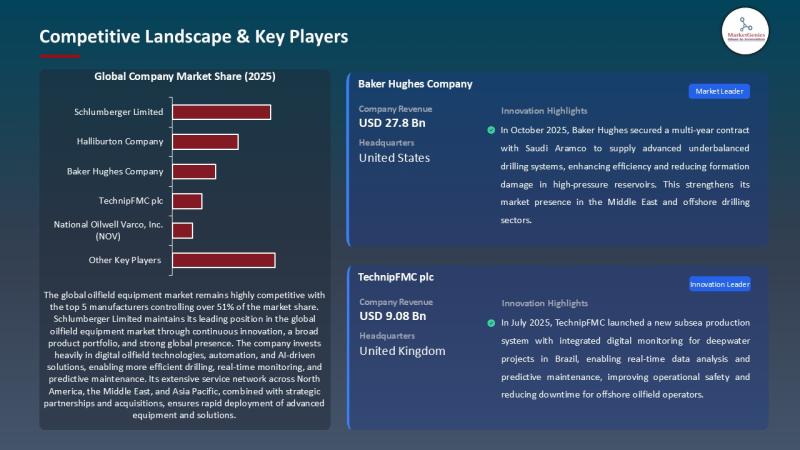
Oilfield Equipment Market hits USD 116.2B in 2025 and grows to USD 156.5B by 203 …
Oilfield Equipment Market | The $156.5B Hardware Backbone of the Global Energy System
Every headline loves clean energy. Yet the global energy mix still demands a brutal truth: oil and gas remain the world's primary supply of heat, mobility, and petrochemicals - and the machines that drill, lift, complete, and produce hydrocarbons continue to define industrial capability.
That's why the Oilfield Equipment Market remains a strategic industry - not a relic.
In 2025,…

Machine Tools Market 2025-2035 | USD 109.9B Growth, CNC & Automation Trends
Machine Tools Market | The $109.9B Intelligence Engine of Global Manufacturing
Factories don't work without machine tools. They shape, cut, drill, grind, and define the physical world around us. Yet most end-products - cars, aircraft parts, electronics housings, surgical devices - never reveal the precision machinery behind them.
The Machine Tools Market is the invisible infrastructure that turns digital models into physical reality.
In 2025, the global Machine Tools Market stands at USD…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…