Press release
Non-Muscle Invasive Bladder Cancer: Clinical Overview, Company Landscape, Therapeutic Evaluation, Treatment Strategies, and Pipeline Analysis | Key Players: Pfizer, Theralase Technologies, Protara The
DelveInsight's, "Non Muscle Invasive Bladder Cancer - Pipeline Insight, 2025" report provides comprehensive insights about 20+ companies and 22+ pipeline drugs in Non Muscle Invasive Bladder Cancer pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.According to DelveInsight, more than 20 leading companies are actively engaged in developing over 25 therapeutic candidates targeting Non-Muscle Invasive Bladder Cancer.
Non Muscle Invasive Bladder Cancer Overview:
Non-muscle invasive bladder cancer (NMIBC) is a type of bladder cancer confined to the inner lining of the bladder, without invasion into the bladder muscle. It represents the most common form of bladder cancer, accounting for approximately 70-80% of all diagnosed cases. In the United States, bladder cancer ranks as the sixth most prevalent cancer, with around 81,000 new cases reported in 2020. The condition occurs more frequently in men than in women, with key risk factors including tobacco use, long-term exposure to industrial chemicals, and chronic bladder irritation or infections. Although NMIBC generally has a more favorable prognosis than muscle-invasive bladder cancer (MIBC), it poses a significant health concern due to its high recurrence rate and potential for progression-emphasizing the need for early detection and timely intervention.
Symptoms of bladder cancer can vary, and in some cases-especially in early stages-individuals may remain asymptomatic. The most common early warning sign is hematuria (blood in the urine), which often appears painlessly and may be visible to the eye or detected microscopically. Other possible symptoms include increased urinary frequency, urgency, or a persistent sensation of incomplete bladder emptying. Patients may also experience dysuria (painful urination), pelvic discomfort, or lower abdominal pain. In more advanced cases, back pain can indicate cancer spread. While these symptoms may also occur in conditions such as urinary tract infections or kidney stones, prompt medical evaluation is crucial. Early diagnosis significantly improves treatment outcomes and long-term prognosis for bladder cancer patients.
Request for a detailed insights report on Non Muscle Invasive Bladder Cancer pipeline insights [https://www.delveinsight.com/report-store/non-muscle-invasive-bladder-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
"Non Muscle Invasive Bladder Cancer Pipeline Insight 2025" report by DelveInsight provides a comprehensive analysis of the ongoing clinical development activities and growth prospects across the Non Muscle Invasive Bladder Cancer Therapeutics Market.
Key Takeaways from the Non Muscle Invasive Bladder Cancer Pipeline Report
*
DelveInsight's Non-Muscle Invasive Bladder Cancer (NMIBC) pipeline report highlights a dynamic and evolving landscape, with over 20 active companies advancing more than 25 therapeutic candidates for NMIBC treatment.
*
On June 12, 2025, the FDA approved ZUSDURI (mitomycin intravesical solution) for adults with recurrent low-grade intermediate-risk NMIBC (LG-IR-NMIBC). This approval, positioning Zusduri as a non-surgical treatment option, marks a significant milestone in improving care for patients with recurrent disease.
*
Leading companies in the NMIBC domain, including Pfizer, Theralase Technologies, Protara Therapeutics, Zhuhai Beihai Biotech Co., Ltd., Vaxiion Therapeutics, Aura Biosciences, Ractigen Therapeutics, Tyra Biosciences, Hoffmann-La Roche, SURGE Therapeutics, CG Oncology, Inc., Trigone Pharma Ltd., and Merck Sharp & Dohme LLC, are actively developing innovative therapies aimed at enhancing the treatment landscape.
*
Notable NMIBC pipeline candidates currently under development include TAR-200, APL-1202, TLD-1433, and several others showing strong potential in improving patient outcomes.
Non Muscle Invasive Bladder Cancer Pipeline Analysis
The report provides insights into:
*
The report provides detailed insights into the key companies that are developing therapies in the Non Muscle Invasive Bladder Cancer Market.
*
The report also evaluates different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Non Muscle Invasive Bladder Cancer treatment.
*
It analyzes the key companies involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
It navigates the emerging drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement, and financing details for future advancement of the Non Muscle Invasive Bladder Cancer market.
Download our free sample page report on Non Muscle Invasive Bladder Cancer pipeline insights [https://www.delveinsight.com/sample-request/non-muscle-invasive-bladder-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Non Muscle Invasive Bladder Cancer Emerging Drugs
TAR-200: Johnson & Johnson Innovative Medicine
TAR-200 is an experimental intravesical drug delivery device developed to provide sustained release of gemcitabine directly into the bladder. It is administered by a healthcare professional using a specialized urinary placement catheter in an outpatient procedure that typically takes under five minutes and does not require anesthesia. The FDA has awarded TAR-200 Breakthrough Therapy Designation for treating adults with BCG-unresponsive, high-risk non-muscle invasive bladder cancer (HR-NMIBC) with carcinoma in situ (CIS) who are either not eligible for or have declined radical cystectomy. The therapy is currently in the Preregistration stage for the treatment of NMIBC.
APL-1202: Asieris Pharmaceuticals
APL-1202, developed by Asieris Pharmaceuticals, is a novel oncology therapy resulting from over a decade of research and the acquisition of patented technology from Johns Hopkins University in the U.S. It is the first-in-class, oral, reversible inhibitor of Methionine Aminopeptidase 2 (MetAP2) and is currently undergoing pivotal Phase III clinical trials for Non-Muscle Invasive Bladder Cancer (NMIBC). Additionally, APL-1202 is being investigated as a neoadjuvant treatment option prior to surgery in patients with Muscle Invasive Bladder Cancer (MIBC). The drug's development has been acknowledged and funded under China's National Major New Drug Innovation Programs during the 12th and 13th Five-Year Plans (2015 and 2018). APL-1202 is presently in Phase III of clinical development for NMIBC.
Non Muscle Invasive Bladder Cancer Companies
More than 20 leading companies are currently developing therapies for Non-Muscle Invasive Bladder Cancer (NMIBC), with Johnson & Johnson Innovative Medicine advancing the most progressed candidate, which is presently in the Preregistration stage.
DelveInsight's report covers around 75+ products under different phases of clinical development like
*
Late stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I) along with the details of
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
Non Muscle Invasive Bladder Cancer pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
*
Intravenous
*
Subcutaneous
*
Oral
*
Intramuscular
Non Muscle Invasive Bladder Cancer Products have been categorized under various Molecule types such as
*
Monoclonal antibody
*
Small molecule
*
Peptide
Download Sample Pages to Get an in-depth Assessment of the Emerging Non Muscle Invasive Bladder Cancer Therapies and Key Companies: Non Muscle Invasive Bladder Cancer Clinical Trials and advancements [https://www.delveinsight.com/report-store/non-muscle-invasive-bladder-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Non Muscle Invasive Bladder Cancer Pipeline Therapeutic Assessment
- Non Muscle Invasive Bladder Cancer Assessment by Product Type
- Non Muscle Invasive Bladder Cancer By Stage
- Non Muscle Invasive Bladder Cancer Assessment by Route of Administration
- Non Muscle Invasive Bladder Cancer Assessment by Molecule Type
Download Non Muscle Invasive Bladder Cancer Sample report to know in detail about the Non Muscle Invasive Bladder Cancer treatment market @ Non Muscle Invasive Bladder Cancer Therapeutic Assessment [https://www.delveinsight.com/sample-request/non-muscle-invasive-bladder-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Content
1. Report Introduction
2. Executive Summary
3. Non Muscle Invasive Bladder Cancer Current Treatment Patterns
4. Non Muscle Invasive Bladder Cancer - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Non Muscle Invasive Bladder Cancer Late-Stage Products (Phase-III)
7. Non Muscle Invasive Bladder Cancer Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Non Muscle Invasive Bladder Cancer Discontinued Products
13. Non Muscle Invasive Bladder Cancer Product Profiles
14. Non Muscle Invasive Bladder Cancer Key Companies
15. Non Muscle Invasive Bladder Cancer Key Products
16. Dormant and Discontinued Products
17. Non Muscle Invasive Bladder Cancer Unmet Needs
18. Non Muscle Invasive Bladder Cancer Future Perspectives
19. Non Muscle Invasive Bladder Cancer Analyst Review
20. Appendix
21. Report Methodology
Request the Sample PDF to Get Detailed Insights About the Non Muscle Invasive Bladder Cancer Pipeline Reports Offerings [https://www.delveinsight.com/report-store/non-muscle-invasive-bladder-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=nonmuscle-invasive-bladder-cancer-clinical-overview-company-landscape-therapeutic-evaluation-treatment-strategies-and-pipeline-analysis-key-players-pfizer-theralase-technologies-protara-the]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Non-Muscle Invasive Bladder Cancer: Clinical Overview, Company Landscape, Therapeutic Evaluation, Treatment Strategies, and Pipeline Analysis | Key Players: Pfizer, Theralase Technologies, Protara The here
News-ID: 4243544 • Views: …
More Releases from ABNewswire
Large Language Model Market to Reach $24.92B by 2031 Driven by Enterprise AI Ado …
Mordor Intelligence has published a new report on the large language model market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Large Language Model Market Outlook
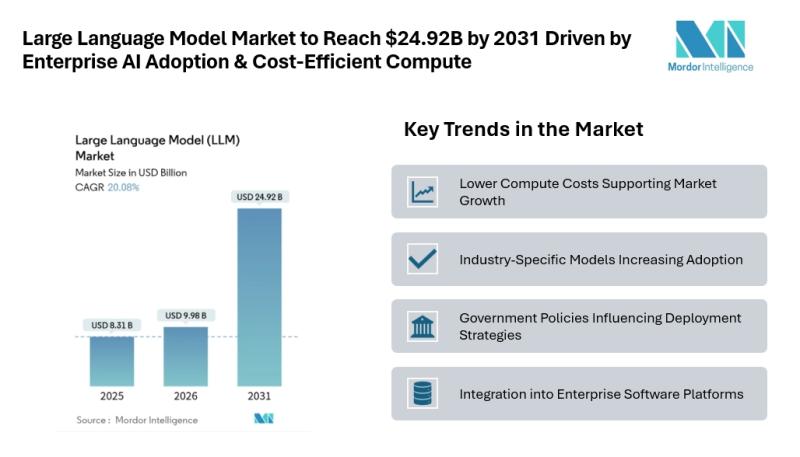
According to Mordor Intelligence, the LLM market size [https://www.mordorintelligence.com/industry-reports/large-language-model-llm-market?utm_source=abnewswire] was valued at USD 8.31 billion in 2025 and is estimated to grow to USD 9.98 billion in 2026, reaching USD 24.92 billion by 2031 at a CAGR of 20.08% during the forecast period.…

Self Employed Tax Software UK: Why Freelancers and Sole Traders Are Switching to …
With Many individuals are seeking software that simplifies tax filing while ensuring full compliance with HMRC requirements. Manual spreadsheets and paper-based calculations are being replaced by real-time, automated systems that give users visibility over their tax position throughout the year. Among the platforms gaining traction is Pie, a UK-based digital tax app built specifically to support self-employed individuals with modern income needs.
LONDON, United Kingdom - February 19, 2026 - Demand…
CivicMail.org Reinvents Postcard Campaigns for Grassroots Advocacy
CivicMail.org aims to bring civic engagement back to basics through the power of pen, paper, and postage.
Image: https://www.abnewswire.com/upload/2026/02/2addd1e9e0381d7e2262e1edbb064123.jpg
CivicMail.org [https://civicmail.org/] has announced its launch to help Americans send real, physical postcards to their elected officials with just a few clicks, delivering personalized messages directly to the desks of decision-makers at the local, state, and federal levels.
Research shows [https://www.concordia.ca/news/stories/2021/09/24/personalized-messages-are-more-likely-to-get-a-response-from-politicians-new-research-finds.html] that physical mail carries more weight with elected officials than petitions, emails, or…

New Children's Story: The Story of Sharin' Bear
A Heartfelt Message Of Courage, Kindness, And The True Meaning Of Giving
A pleasant new story for children, The Story of Sharin' Bear by Sharon Woods , introduces families to a lovable little cub whose journey of bravery and compassion changes him into a representation of sharing for children globally.
Entrenched in adventure, innocence, and emotional growth, this uplifting tale offers an unforgettable reminder that even the smallest acts of kindness can…
More Releases for Invasive
Non-Invasive Invasive Laser Lipolysis Machine Market Size, Share and Growth Repo …
On May 9, 2025, Exactitude Consultancy., Ltd. released a research report titled "Non-Invasive Invasive Laser Lipolysis Machine Market". This report covers the global Non-Invasive Invasive Laser Lipolysis Machine market sales, sales volume, price, market share, ranking of major companies, etc., and provides a detailed analysis by region, country, product type, and application. It also forecasts the market size of automotive kick sensors based on market patterns from 2020 to 2034…
Aesthetic Medicine Market, By Procedure Type (Invasive Procedures, Non-invasive …
Aesthetic medicine is a type of cosmetic procedure which is used in the treatment to improve scars, wrinkles, liver spots, cellulite, unwanted hair, excess fat and others that will help in augmenting the physical appearance of the patient using minimally invasive and non-invasive procedure.
View Detailed Report: https://www.databridgemarketresearch.com/reports/global-aesthetic-medicine-market
Data Bridge Market Research analyses that the aesthetic medicine market is expected to reach the value of USD 26.68 Billion by the year 2029,…
Minimally Invasive Surgical Instruments Market - Driving Surgical Excellence wit …
Newark, New Castle, USA: The "Minimally Invasive Surgical Instruments Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Minimally Invasive Surgical Instruments Market: https://www.growthplusreports.com/report/minimally-invasive-surgical-instruments-market/7672
This latest report researches the…
Minimally Invasive And Non Invasive Product And Service Market Market Trends Ana …
The research report on the Global Minimally Invasive And Non-Invasive Product And Service Market offers numerous market frameworks, including market size, portion, trends, growth path, value, and factors that affect the current market dynamics throughout the projected period of 2022-2030. Most importantly, along with their market shares, this research also includes the most essential recent strategies used by major companies. The market for minimally invasive and non-invasive goods and services…
Micro-Invasive Glaucoma Implants Micro-Invasive Glaucoma Implants
Global Micro-Invasive Glaucoma Implants Market Definition: Micro-invasive glaucoma implants is performed for the treatment of the open- angle glaucoma and is done through an ab- interno approach. It is very safe and provides faster recovery as compared to the traditional methods. They usually lower the intraocular by increasing the flow or reducing the production of the aqueous humor. Increasing cases of the glaucoma worldwide is the major factor fueling the…
Respiratory Humidification Market for Invasive & Non-Invasive Ventilation | Late …
Researchmoz added Most up-to-date research on "Respiratory Humidification Market (Controller & Consumables) for Invasive & Non-Invasive Ventilation" to its huge collection of research reports.
Respiratory humidification is a method of artificial warming and humidifying of respiratory gas for mechanically ventilated patients. It is a method of artificially conditioning respiratory gas for the patient during therapy. Humidifiers are used in respiratory and acute care (RAC) and for the treatment of sleep apnea.…
