Press release
Sterilized Packaging Market to Reach USD 629.50 Million by 2031 Top 20 Company Globally
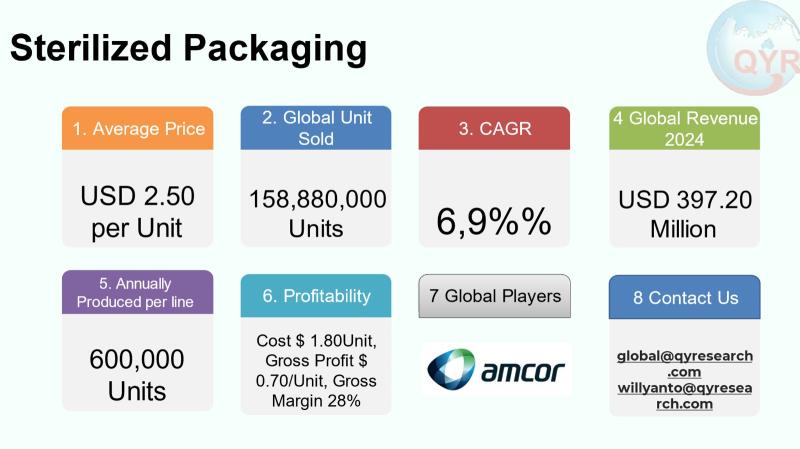
The sterilized packaging industry provides primary and secondary containment designed to maintain sterility from manufacture through storage and use for medical devices, pharmaceuticals, biologics, and aseptically processed foods and beverages. It covers a broad set of materials and formats including flexible pouches and films, rigid plastics, glass vials and ampoules, metal containers, and nonwoven sterilization wraps together with the combination of barrier layers, sterilizable seals, and compatible sterilization processes (steam, EO, gamma, electron-beam, or aseptic filling). The industry sits at the intersection of highly regulated healthcare/pharma supply chains and food safety systems, and demand is driven by rising surgical procedures, biologics and injectable therapies, growth in ready-to-drink and preservative-free foods, and by stricter infection-control regulation across regions.The global sterilized packaging market value in 2024 amounted to USD 397.20 million with an expected compound annual growth rate of 6.9% through 2031, reaching market size USD 629.50 million by 2031. An average selling price is USD 2.50 per unit, implies to a total of 158,880,000 unit sold globally in 2024. Factory profit margin is 28% equal to factory gross profit of USD 0.70 per unit and cost of goods of USD 1.80 per unit. A COGS breakdown is raw materials, conversion/processing and sterilization, packaging/secondary packing and testing and overhead/logistics the remainder. A single line full machine production capacity is around 600,000 unit per line per year. Downstream industry demand is concentrated in healthcare/pharmaceuticals, food & beverage aseptic formats, and other segments.
Latest Trends and Technological Developments
The sterilized packaging sector is being reshaped by automation of aseptic fill-finish and inline sterilization, the rise of single-use systems in bioprocessing, materials innovation for recyclability and reduced VOCs, and digital quality controls (inline vision, traceability with RFID/serialization and cold-chain sensors). Recent industry news illustrates these themes: Sidel announced a full aseptic PET line installation emphasizing flexibility and FDA-grade aseptic performance (press release, May 2025), showing OEM investment into flexible aseptic beverage lines and multipack adaptability. BioProcess Internationals June 2025 coverage highlighted fill-finish and Annex 1 compliance pressures pushing fill-finish facilities toward more closed-system, higher-automation sterile production. Market research publishers continue to report strong growth in aseptic and sterilized packaging equipment demand driven by biologics and preservative-free foods. These developments collectively point toward accelerating capital investment in automated aseptic lines, closer collaboration between converters and pharmaceutical OEMs on sterilization validation, and greater attention to sustainable barrier materials.
Lush Cosmetics purchases custom-molded, biodegradable starch-based containers from EcoLogic Solutions for their signature solid shampoo bars. The unique, ergonomic, and visually distinct packaging is a core part of their brand identity and in-store experience, justifying a premium price. Lush pays approximately $1.25 per unit for these stylized containers, a significant increase over standard cosmetic jars, because the innovative design reduces the need for secondary boxing and directly communicates their ethical and environmental values to the consumer at the point of sale.
The stylized, vacuum-formed PET plastic clamshell packaging, sourced from Placon Corporation, is used by Microsoft for their Xbox Wireless Controller. This specific packaging is applied on the assembly line, with each controller securely nestled in the precisely molded plastic, amounting to a cost of $0.85 per unit. The package is designed not only for high-impact, theft-deterrent retail display but also to be easily opened without scissors, enhancing the unboxing experience and reinforcing the premium nature of the product for the end-user.
Asia remains one of the fastest growing regions for sterilized packaging due to rising healthcare expenditure, expanding pharmaceutical manufacturing (including contract manufacturing and biosimilars), growth in ready-to-drink and preservative-free beverages, and growing domestic converters scaling for export. China and India are major volume centers for standard sterilized consumables and rigid containers, with China also expanding aseptic carton and PET aseptic capacity for beverages. Japan, South Korea, Taiwan, Singapore, and parts of the Middle East function as centers for higher-spec pharmaceutical packaging and innovative materials. Across Asia, cost sensitivity encourages adoption of higher-speed, lower-cost formats for disposables, but regulatory tightening (GMP, Annex 1 analogues) is driving upgrades in cleanroom investment and supplier qualification protocols. Regional equipment OEM activity and local converter expansion have been visible in 2024 to 2025, with both international OEMs and local machinery builders supplying lines tailored to regional beverage and pharmaceutical clients.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart)
https://www.qyresearch.com/sample/3431451
Sterilized Packaging by Type:
Plastics Sterilized Packaging
Glass Sterilized Packaging
Metal Sterilized Packaging
Nonwoven Sterilized Packaging
Others
Sterilized Packaging by Product Category:
Peel Pouches
Thermoformed Trays
Sterilization Wraps
Header Bags
Others
Sterilized Packaging by Market Segment:
Porous Packaging
Non-Porous Packaging
Sterilized Packaging by Product Division:
Steam Sterilization Compatible
EO Sterilization Compatible
VHP/Plasma Sterilization Compatible
Radiation Sterilization Compatible
Others
Sterilized Packaging by Shape:
Pouches and Header Bags
Trays and Rigid Containers
Ampoules and Vials
Cartons and Bottles
Others
Sterilized Packaging by Application:
Pharmaceutical
Medical Instruments
Medical Implants
Others
Global Top 20 Key Companies in the Sterilized Packaging Market
Amcor
DuPont
3M
Berry Global
Wihuri Group
Tekni-Plex
West Pharmaceutical
Placon Corporation
SCHOTT
Gerresheimer
Riverside Medical Packaging
Oliver-Tolas
Technipaq
Baxter Healthcare
Anqing Kangmingna Packaging
Duomed (Palex)
Röchling Medical
Euronda
Wipak Group
Nelipak
Regional Insights
In ASEAN, sterilized packaging growth is led by beverage aseptic formats, hospital consumables, and rising pharma fill-finish activity in hubs such as Singapore and Thailand; Indonesias large food & beverage market and rising domestic healthcare demand make it a high-growth market for pouches, flexible sterilization wraps, and primary sterile packaging for domestic medtech manufacturing. Indonesia is increasingly a destination for downstream packaging contracts because of local consumption scale and competitive conversion costs, but some high-barrier pharmaceutical packaging still relies on imports or regionalized production in Singapore and Malaysia. Key regional dynamics include the need for localized sterilization validation capability (gamma/e-beam/ETO access), logistics for cold chain and sterile handling, and regulatory harmonization to streamline cross-border supplier qualification. Market players targeting ASEAN often combine regional converting plants with service offerings for sterilization validation and technical support to win hospital and pharmaceutical contracts.
The industry faces multiple headwinds: first, regulatory and validation complexity (various Annex-style requirements, bioburden control, serialization and traceability) increases time-to-market and qualification costs; second, capital intensity for aseptic and fill-finish equipment and for in-house sterilization (gamma/E-beam/EO) raises barrier to entry; third, raw-material volatility (polymers, specialty films, barrier laminates, glass) compresses margins and complicates forecasting; fourth, sustainability pressures require materials innovation while maintaining barrier/sterility performance, a difficult materials science tradeoff; and fifth, talent shortages for cleanroom operations and sterile process engineers slow capacity scale-up in many growth markets. These challenges push manufacturers to adopt risk-sharing partnerships, contract manufacturing models, and to invest in automation and inline quality systems.
Manufacturers and investors should prioritize modular aseptic and fill-finish capacity (to reduce changeover and shorten validation cycles), partnerships with sterilization service providers (to avoid heavy capital in gamma/ETO), digital traceability and quality monitoring to reduce recalls and simplify regulators inspections, and active material R&D for recyclable or mono-polymer barrier solutions. For players focused on ASEAN and Indonesia, local technical support, regional warehousing for validated sterile goods, and assistance with country-level regulatory dossiers are strategic differentiators. Contract manufacturers should explore blended business models that combine routine sterilized disposables production with niche higher-margin pharma fill-finish services to balance volume and margin.
Product Models
Sterilized packaging refers to specialized packaging materials and systems designed to maintain the sterility of medical, pharmaceutical, and food products. These packaging types undergo stringent sterilization processes to prevent microbial contamination and ensure product safety.
Plastics sterilized packaging refers to packaging materials made from polymers such as polyethylene, polypropylene, or PET that are designed to maintain product sterility after processes like gamma irradiation, ethylene oxide, or steam sterilization. Notable products include:
Tyvek® Medical Packaging DuPont: High-strength, breathable packaging for sterilized medical instruments.
SteriPouch Oliver Healthcare Packaging: Heat-sealable plastic pouches used for sterile surgical devices.
Sterimed Laminates Sterimed Group: Multilayer plastic film designed for steam and EO sterilization.
MedLock Pack Technipaq: Polypropylene-based sterile pouches for single-use medical items.
ProPEEL Film Amcor Flexibles: Peelable sterile film used for disposable medical packaging.
Glass sterilized packaging involves the use of pre-sterilized or sterilizable glass containers, including vials, ampoules, and syringes, primarily used for injectable drugs and biopharmaceuticals. Examples include:
DWK Life Sciences Vials DWK Life Sciences: Sterile borosilicate vials for laboratory use.
Nipro Sterile Glass Ampoules Nipro Corporation: Pre-sealed glass ampoules for sterile liquid drugs.
Gerresheimer Gx RTF® Gerresheimer AG: Ready-to-fill sterile glass containers for biotech drugs.
Bormioli Pharma Glass Vials Bormioli Pharma: Sterilized glass containers for parenteral applications.
Shandong PG Glass Vials Shandong PG: Chinese-made sterile glass containers for injectable solutions
Metal sterilized packaging uses stainless steel, aluminum, or other alloys to encase surgical instruments and medical tools during sterilization cycles. Notable products include:
KLS Martin Sterilization Box KLS Martin Group: Stainless steel packaging for surgical tool storage.
CaseMed Aluminum Case Case Medical: Lightweight anodized aluminum sterile cases.
Aygun Instrument Container Aygun Surgical: Autoclavable stainless packaging for medical devices.
Medline Reusable Metal Container Medline Industries: Metal boxes for hospital sterilization cycles.
Skytron Instrument Case Skytron: Metal packaging designed for repeated sterilization cycles.
Nonwoven sterilized packaging consists of fiber-based synthetic fabrics engineered to allow sterilant penetration while maintaining a microbial barrier post-sterilization. Commonly used as sterilization wraps in hospitals, they are lightweight, breathable, and suitable for steam. Notable products include:
Medline CSR Wrap Medline Industries: Nonwoven wrap used for surgical instrument sterilization.
Cardinal Health Sterile Wrap Cardinal Health: Breathable wrap that allows sterilant penetration.
SteriTex Wrap Dupont: Nonwoven fabric wrap offering microbial barrier protection.
Hogy Medical Wrap Hogy Medical Japan: Japanese sterile wrap for hospital instruments.
Dynarex Sterilization Wrap Dynarex Corporation: Nonwoven medical wrap ensuring sterile protection.
The sterilized packaging industry sits in steady growth driven by healthcare expansion, biologics and injectable therapies, and aseptic food & beverage demand; the 2024 base of USD 397.20 million and a 6.9% CAGR to 2031 imply meaningful cumulative growth, but that growth requires significant capital investment and compliance capability. Suppliers who combine validated sterilization processes, automation, regional manufacturing footprints (particularly in Asia and ASEAN), and credible sustainability roadmaps will capture the higher value segments. Investors and strategic buyers should expect a mix of high-throughput commodity volumes alongside high-margin specialized pharmaceutical formats, and must weight regulatory and capital intensity in their financial models.
Investor Analysis
This research highlights three investor-relevant takeaways. First, market size and steady CAGR with diverse end markets (pharma and food) create portfolio resilience: investors can capture defensive healthcare demand and growth in aseptic food formats. Second, capital intensity and regulatory barriers create scalable moat opportunities for established converters companies with validated sterilization facilities and qualified customer lists can enjoy durable contracts and higher switching costs. Third, technology and materials shift (automation, single-use bioprocessing, recyclable barriers) create event-driven upside: targeted investments in OEMs or converters that adopt automation and sustainable barrier tech can realize premium valuations. In practical terms, investors should evaluate potential targets on four axes: validated sterile capacity and certifications, customer concentration in pharma vs food, modular automation (reduces validation time/cost), and raw-material sourcing resilience. These criteria help prioritize targets that can deliver stable cash flows, margin improvement through automation, and growth via regional expansion in Asia/ASEAN.
Request for Pre-Order Enquiry On This Report
https://www.qyresearch.com/customize/3431451
5 Reasons to Buy This Report
Comprehensive market sizing anchored to 2024 and forward growth to 2031, with regional focus on Asia and ASEAN.
Detailed manufacturing economics including price ranges, COGS breakdowns, factory gross profit and margin guidance, and per-line capacity benchmarks.
Actionable regional insight for Asia and ASEAN covering demand drivers, regulatory considerations and supply-chain implications.
Technology and trend analysis with recent industry examples and dated news items demonstrating real equipment deployments and regulatory drivers.
Strategic recommendations and investor due diligence checklist linking operational metrics to valuation levers.
5 Key Questions Answered
What is the 2024 market size and projected CAGR through 2031 for sterilized packaging?
What are realistic price ranges, units-sold scale, and COGS breakdowns across typical sterilized packaging SKUs?
How do Asia and ASEAN markets differ in demand mix, regulatory pressure, and local capacity?
Which technological and regulatory trends (automation, single-use, Annex-style compliance) will most influence capital deployment and margins?
Who are the leading global players, and what capabilities or assets are most valuable to acquire or partner with?
Chapter Outline
Chapter 1: Introduces the report scope of the report, executive summary of different market segments (by region, product type, application, etc), including the market size of each market segment, future development potential, and so on. It offers a high-level view of the current state of the market and its likely evolution in the short to mid-term, and long term.
Chapter 2: key insights, key emerging trends, etc.
Chapter 3: Manufacturers competitive analysis, detailed analysis of the product manufacturers competitive landscape, price, sales and revenue market share, latest development plan, merger, and acquisition information, etc.
Chapter 4: Provides profiles of key players, introducing the basic situation of the main companies in the market in detail, including product sales, revenue, price, gross margin, product introduction, recent development, etc.
Chapter 5 & 6: Sales, revenue of the product in regional level and country level. It provides a quantitative analysis of the market size and development potential of each region and its main countries and introduces the market development, future development prospects, market space, and market size of each country in the world.
Chapter 7: Provides the analysis of various market segments by Type, covering the market size and development potential of each market segment, to help readers find the blue ocean market in different market segments.
Chapter 8: Provides the analysis of various market segments by Application, covering the market size and development potential of each market segment, to help readers find the blue ocean market in different downstream markets.
Chapter 9: Analysis of industrial chain, including the upstream and downstream of the industry.
Chapter 10: The main points and conclusions of the report.
Related Report Recommendation
Global Sterilized Packaging Market Research Report 2025
https://www.qyresearch.com/reports/3431451/sterilized-packaging
Global Sterilized Packaging Sales Market Report, Competitive Analysis and Regional Opportunities 2025-2031
https://www.qyresearch.com/reports/4844904/sterilized-packaging
Global Sterilized Packaging Market Outlook, InDepth Analysis & Forecast to 2031
https://www.qyresearch.com/reports/4919210/sterilized-packaging
Sterilized Packaging - Global Market Share and Ranking, Overall Sales and Demand Forecast 2025-2031
https://www.qyresearch.com/reports/5289885/sterilized-packaging
Global Sterilized Medical Packaging Market Research Report 2025
https://www.qyresearch.com/reports/4328536/sterilized-medical-packaging
Global Pre-Sterilized & Ready-to-Use Primary Packaging Market Research Report 2025
https://www.qyresearch.com/reports/4260229/pre-sterilized---ready-to-use-primary-packaging
Global Medical Sterilizing Packing Bag Market Research Report 2025
https://www.qyresearch.com/reports/3574205/medical-sterilizing-packing-bag
Global Radiation Sterilized Medical Packaging Market Research Report 2025
https://www.qyresearch.com/reports/4195445/radiation-sterilized-medical-packaging
Global Pre-sterilized - Ready to Use Pharmaceutical Packaging Market Research Report 2025
https://www.qyresearch.com/reports/4401169/pre-sterilized---ready-to-use-pharmaceutical-packaging
Global Medical Sterilizing Packing Bag Market Research Report 2025
https://www.qyresearch.com/reports/3574205/medical-sterilizing-packing-bag
Contact Information:
Tel: +1 626 2952 442 (US) ; +86-1082945717 (China)
+62 896 3769 3166 (Whatsapp)
Email: willyanto@qyresearch.com; global@qyresearch.com
Website: www.qyresearch.com
About QY Research
QY Research has established close partnerships with over 71,000 global leading players. With more than 20,000 industry experts worldwide, we maintain a strong global network to efficiently gather insights and raw data.
Our 36-step verification system ensures the reliability and quality of our data. With over 2 million reports, we have become the world's largest market report vendor. Our global database spans more than 2,000 sources and covers data from most countries, including import and export details.
We have partners in over 160 countries, providing comprehensive coverage of both sales and research networks. A 90% client return rate and long-term cooperation with key partners demonstrate the high level of service and quality QY Research delivers.
More than 30 IPOs and over 5,000 global media outlets and major corporations have used our data, solidifying QY Research as a global leader in data supply. We are committed to delivering services that exceed both client and societal expectations.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Sterilized Packaging Market to Reach USD 629.50 Million by 2031 Top 20 Company Globally here
News-ID: 4243324 • Views: …
More Releases from QY Research
Top 30 Indonesian Food and Beverages Public Companies Q3 2025 Revenue & Performa …
1) Overall companies performance (Q3 2025 snapshot)
PT Indofood Sukses Makmur Tbk (INDF) Large diversified food parent with beverage exposure
PT Mayora Indah Tbk (MYOR) Coffee, tea, RTD beverages & snacks
PT Ultrajaya Milk Industry & Trading Company Tbk (ULTJ) Milk & drinks
PT Indofood CBP Sukses Makmur Tbk (ICBP) Integrated processed food & beverage mix
PT Garudafood Putra Putri Jaya Tbk (GOOD) Snack foods &…
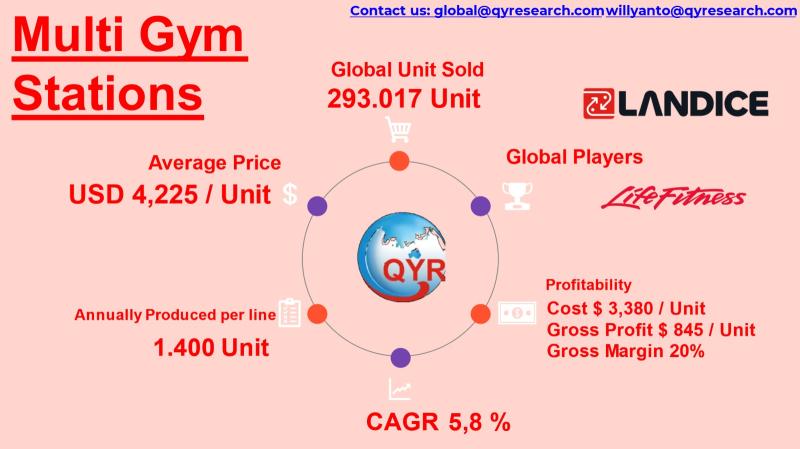
Connected Strength Training: The New Growth Engine for the Gym Equipment Industr …
Multi station gyms (also called multi-function home or commercial strength stations) integrate several resistance training modulespress, lat pull-down, leg curl/extension, cable crossover, and functional trainer into one compact system.
Equipment is widely adopted across home fitness, corporate wellness, apartment gyms, hotel fitness centers, schools, and commercial fitness clubs.
Demand is driven by rising health awareness, urban space constraints, and preference for all-in-one solutions that reduce footprint and capex compared to multiple single…

Low Sodium, High Growth: The Next Big Opportunity in Food Reformulation
Food grade salt replacement ingredients are formulated compounds designed to partially or fully substitute sodium chloride in processed foods while maintaining taste, preservation, and texture functionality.
These solutions are increasingly adopted due to global sodium-reduction mandates, rising hypertension prevalence, and clean-label food reformulation.
Products typically include potassium chloride blends, yeast extracts, amino acid systems, mineral salts, fermentation-derived flavor enhancers, and encapsulated masking technologies.
Applications cover snacks, bakery, processed meat, instant noodles, sauces, ready…

High-Margin Specialty Chemicals: Investment Guide to Biofuel Lubricity Boosters
Biofuel lubricity boosting agents are specialty chemical additives blended into biodiesel, renewable diesel, HVO, and low-sulfur fuels to restore or enhance fuel lubricity and protect pumps, injectors, and fuel systems from wear.
With tightening sulfur regulations and increased biofuel blending mandates, natural lubricity has declined, making lubricity improvers a mandatory additive class across most commercial biofuel blends.
The industry sits at the intersection of:
Specialty fuel additives
Renewable fuels and biodiesel production
Refinery blending operations
Engine…
More Releases for Pack
DQ PACK Leads In Sustainable Packaging With Eco-Friendly Recyclable Bags At PACK …
DQ PACK, a China Eco Friendly Recyclable Packaging Bag Supplier(https://www.dqpack.com/), showcased its latest innovations in sustainable packaging at the highly anticipated PACK EXPO International. As a global leader in flexible packaging solutions, the company is highlighting its new line of high-performance, eco-friendly recyclable bags. These next-generation bags reflect DQ PACK's strong commitment to environmental stewardship and its proactive approach to meeting the rising global demand for responsible packaging. By offering…
Correct Pack to Showcase Coding and Marking Solutions at PACK EXPO Las Vegas 202 …
Coding and marking solutions by Correct Pack will include the CP9000P Pigment Printer and CP6005U UV Laser, to be showcased at PACK EXPO Las Vegas 2025.
Zhuhai, Guangdong, China - Correct Pack Technology Company will participate in PACK EXPO Las Vegas 2025, taking place from September 29 to October 1 at the Las Vegas Convention Center. The company will present its advanced coding and marking equipment at Booth SU-36028, engaging with…
Transformative Trends Impacting the Gel Ice Pack Market Landscape: Innovation in …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Gel Ice Pack Market Size By 2025?
In recent years, the market size for gel ice packs has seen a significant expansion. The market is projected to rise from $12.5 billion in 2024 to $14.69 billion in 2025, with a compound annual growth rate (CAGR)…
VITAMIST® Revolutionizes Convenience with the Launch of New Multi-Pack and Max- …
VITAMIST®, the pioneers of oral vitamin sprays, is thrilled to announce the launch of its innovative Multi-Pack and Max-Pack product concepts, designed to provide tailored wellness solutions for every lifestyle. These carefully curated packs are a game-changer for those seeking convenience, variety, and effectiveness in their health routines.
The Multi-Pack range includes four unique offerings: Immunity, Travel, Fitness, and Beauty, each containing three targeted vitamin sprays that address specific…
EV Battery Pack Cooling System Market Keeping it Cool: The EV Battery Pack Cooli …
Global EV battery Pack Cooling System Market Worth $8.09 Bn by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global EV battery Pack Cooling System Market- (Vehicle Type (Passenger Vehicle, and Commercial Vehicle), By Propulsion Type (Battery Electric Vehicle, Hybrid Electric Vehicle and Plug-in Hybrid Electric Vehicle), By System Type (Air Cooling System and Liquid Cooling…
Smart Intelligent ENG Battery Pack | NEOSEMITECH
NEO SEMITECH NEO Q ENG Battery Pack is a newly developed smart intelligent ENG Battery Pack product by NEO SEMITECH CO., LTD. in South Korea. The biggest differentiated excellence of NEO Q ENG Battery Pack is the battery level indicator that guarantees the utmost user convenience. The product is designed with enhanced grip considering user convenience, and the switch is also placed visibly on the front side for the sake…