Press release
Atopic Dermatitis Clinical, Companies, Therapy Assessment, Therapies, Pipeline | Vanda Pharmaceuticals, TechnoDerma Medicines, Asana BioSciences, Artax Biopharma, Corvus Pharmaceuticals, Yuhan, BioVer
DelveInsight's, "Atopic Dermatitis - Pipeline Insight, 2025" report provides comprehensive insights about 100+ companies and 120+ pipeline drugs in Atopic Dermatitis pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.DelveInsight's analysis indicates that the Atopic Dermatitis pipeline features over 100 active companies dedicated to advancing more than 120 therapeutic candidates for the treatment of Atopic Dermatitis.
Atopic Dermatitis Overview:
Atopic Dermatitis (AD), widely referred to as eczema, is a chronic inflammatory skin condition that typically appears in early childhood but can emerge at any stage of life, often persisting or recurring over time. The word dermatitis originates from "derm" (skin) and "itis" (inflammation), emphasizing the condition's inflammatory nature. AD is characterized by itching, redness, and rashes, which result from a complex interplay of genetic susceptibility, immune system hyperactivity, environmental triggers, allergens, and infections.
Almost 50% of people with moderate-to-severe eczema also experience other atopic conditions, such as asthma, allergic rhinitis (hay fever), or food allergies. It is the most common chronic skin disease in children. The defining feature is dry, itchy skin that can progress into red, inflamed lesions during flare-ups. A range of internal and external triggers can provoke these flares, leading to inflammation, increased blood flow, and severe itching. This causes a persistent itch-scratch cycle, which may result in skin thickening (lichenification), discoloration, dryness, and secondary infections.
The pathophysiology of AD is complex, involving skin barrier defects, immune dysregulation, IgE-mediated hypersensitivity, and environmental influences. Mutations in the filaggrin (FLG) gene are strongly linked to more severe forms of AD, as they promote water loss, elevate skin pH, and increase dehydration. Other genetic variations may further weaken the skin barrier, heightening susceptibility. An imbalance between Th2 and Th1 cytokines contributes to excessive IgE production and abnormal immune activity. On a microscopic scale, spongiosis (fluid accumulation between skin cells) facilitates the infiltration of inflammatory mediators. Various dendritic cells-including Langerhans cells, inflammatory dendritic epidermal cells, and plasmacytoid dendritic cells-play vital roles in driving disease progression.
Request for a detailed insights report on Atopic Dermatitis pipeline insights [https://www.delveinsight.com/report-store/atopic-dermatitis-ad-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
"Atopic Dermatitis Pipeline Insight 2025" report by DelveInsight provides a comprehensive analysis of the ongoing clinical development activities and growth prospects across the Atopic Dermatitis Therapeutics Market.
Key Takeaways from the Atopic Dermatitis Pipeline Report
*
DelveInsight's Atopic Dermatitis Pipeline Report highlights a vibrant and evolving landscape, with over 100 active companies developing more than 120 investigational therapies aimed at advancing Atopic Dermatitis (AD) treatment.
*
In September 2025, Incyte Corporation's topical Opzelura (ruxolitinib cream 1.5 %) was approved by the FDA for children aged 2 to 11 with mild-to-moderate AD whose disease is not adequately controlled with topical therapies.
*
In October 2025, Arcutis Biotherapeutics's topical Zoryve (roflumilast cream 0.05 %) received FDA approval for children aged 2 to 5 with mild-to-moderate AD.
*
In October 2025, Eli Lilly and Company reported promising long-term data for its biologic Ebglyss (lebrikizumab) in adolescents with moderate-to-severe AD, and has submitted label-expansion data to the FDA.
*
The FDA has extended the PDUFA review deadline to September 19, 2025, for the 0.75% ruxolitinib cream supplemental application targeting children aged 2-11 years with mild-to-moderate AD, allowing additional time to review updated chemistry, manufacturing, and control (CMC) data.
*
In December 2024, the FDA approved Nemluvio, the first IL-31 receptor A-targeting therapy, for patients aged 12 and older with moderate-to-severe AD not adequately controlled by topical corticosteroids or calcineurin inhibitors, offering relief from itch and inflammation.
*
Similarly, in September 2024, Ebglyss received FDA approval for patients aged 12 and above with moderate-to-severe AD. This once-monthly injectable biologic, which neutralizes IL-13, demonstrated strong efficacy in clinical trials involving over 1,000 participants.
*
Leading companies such as Vanda Pharmaceuticals, TechnoDerma Medicines, Asana BioSciences, Artax Biopharma, Corvus Pharmaceuticals, Yuhan, BioVersys, Rubedo Life Sciences, Innocare Pharma, Apogee Therapeutics, Celldex Therapeutics, Aclaris Therapeutics, and Astria Therapeutics are actively working to reshape the AD treatment landscape through innovative drug development.
*
Prominent pipeline candidates across various stages include ICP-332, APG777, Barzolvolimab, ATI-2138, STAR-0310, and several others showing promising therapeutic potential.
Atopic Dermatitis Pipeline Analysis
The report provides insights into:
*
The report provides detailed insights into the key companies that are developing therapies in the Atopic Dermatitis Market.
*
The report also evaluates different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Atopic Dermatitis treatment.
*
It analyzes the key companies involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
It navigates the emerging drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement, and financing details for future advancement of the Atopic Dermatitis market.
Download our free sample page report on Atopic Dermatitis pipeline insights [https://www.delveinsight.com/sample-request/atopic-dermatitis-ad-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Atopic Dermatitis Emerging Drugs
ICP-332 - InnoCare Pharma
ICP-332 is a potent and highly selective TYK2 inhibitor under development for treating several T-cell-mediated autoimmune disorders, including atopic dermatitis (AD), vitiligo, and inflammatory bowel disease (IBD).
TYK2, a non-receptor tyrosine kinase belonging to the JAK family, is a crucial component of the JAK-STAT signaling pathway, which regulates immune and inflammatory responses. By targeting this pathway, ICP-332 offers broad therapeutic potential across multiple inflammatory conditions. The drug is currently in Phase III clinical trials for atopic dermatitis.
APG777 - Apogee Therapeutics
APG777 is a novel, long-acting monoclonal antibody administered subcutaneously, specifically engineered to target interleukin-13 (IL-13) for the treatment of atopic dermatitis (AD).
In preclinical studies, APG777 exhibited comparable or superior efficacy to lebrikizumab in inhibiting IL-13 signaling. Results from a 12-month Phase I trial demonstrated an extended half-life of 77 days, a strong safety profile, and favorable pharmacodynamic outcomes. A single dose achieved near-complete inhibition of pSTAT6 and sustained suppression of TARC levels for up to one year. The therapy is currently being evaluated in Phase II clinical trials for atopic dermatitis.APG777 is a novel, long-acting monoclonal antibody administered subcutaneously, specifically engineered to target interleukin-13 (IL-13) for the treatment of atopic dermatitis (AD).
Atopic Dermatitis Companies
Over 100 prominent companies are actively engaged in developing therapies for Atopic Dermatitis. Among them, InnoCare Pharma is one of the companies with a drug candidate that has reached the most advanced stage of development-Phase III clinical trials.
DelveInsight's report covers around 75+ products under different phases of clinical development like
*
Late stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I) along with the details of
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
Atopic Dermatitis pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
*
Intravenous
*
Subcutaneous
*
Oral
*
Intramuscular
Atopic Dermatitis Products have been categorized under various Molecule types such as
*
Monoclonal antibody
*
Small molecule
*
Peptide
Download Sample Pages to Get an in-depth Assessment of the Emerging Atopic Dermatitis Therapies and Key Companies: Atopic Dermatitis Clinical Trials and advancements [https://www.delveinsight.com/report-store/atopic-dermatitis-ad-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Atopic Dermatitis Pipeline Therapeutic Assessment
- Atopic Dermatitis Assessment by Product Type
- Atopic Dermatitis By Stage
- Atopic Dermatitis Assessment by Route of Administration
- Atopic Dermatitis Assessment by Molecule Type
Download Atopic Dermatitis Sample report to know in detail about the Atopic Dermatitis treatment market @ Atopic Dermatitis Therapeutic Assessment [https://www.delveinsight.com/sample-request/atopic-dermatitis-ad-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Content
1. Report Introduction
2. Executive Summary
3. Atopic Dermatitis Current Treatment Patterns
4. Atopic Dermatitis - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Atopic Dermatitis Late-Stage Products (Phase-III)
7. Atopic Dermatitis Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Atopic Dermatitis Discontinued Products
13. Atopic Dermatitis Product Profiles
14. Atopic Dermatitis Key Companies
15. Atopic Dermatitis Key Products
16. Dormant and Discontinued Products
17. Atopic Dermatitis Unmet Needs
18. Atopic Dermatitis Future Perspectives
19. Atopic Dermatitis Analyst Review
20. Appendix
21. Report Methodology
Request the Sample PDF to Get Detailed Insights About the Atopic Dermatitis Pipeline Reports Offerings [https://www.delveinsight.com/report-store/atopic-dermatitis-ad-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=atopic-dermatitis-clinical-companies-therapy-assessment-therapies-pipeline-vanda-pharmaceuticals-technoderma-medicines-asana-biosciences-artax-biopharma-corvus-pharmaceuticals-yuhan-biover]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Atopic Dermatitis Clinical, Companies, Therapy Assessment, Therapies, Pipeline | Vanda Pharmaceuticals, TechnoDerma Medicines, Asana BioSciences, Artax Biopharma, Corvus Pharmaceuticals, Yuhan, BioVer here
News-ID: 4241451 • Views: …
More Releases from ABNewswire
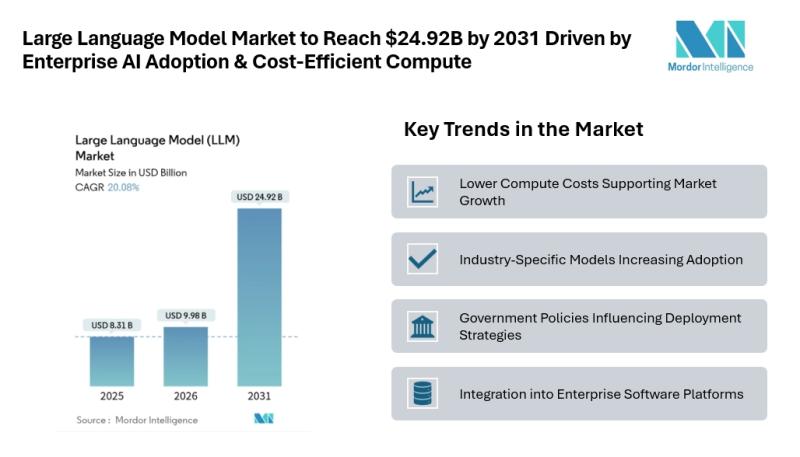
Large Language Model Market to Reach $24.92B by 2031 Driven by Enterprise AI Ado …
Mordor Intelligence has published a new report on the large language model market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Large Language Model Market Outlook
According to Mordor Intelligence, the LLM market size [https://www.mordorintelligence.com/industry-reports/large-language-model-llm-market?utm_source=abnewswire] was valued at USD 8.31 billion in 2025 and is estimated to grow to USD 9.98 billion in 2026, reaching USD 24.92 billion by 2031 at a CAGR of 20.08% during the forecast period.…

Self Employed Tax Software UK: Why Freelancers and Sole Traders Are Switching to …
With Many individuals are seeking software that simplifies tax filing while ensuring full compliance with HMRC requirements. Manual spreadsheets and paper-based calculations are being replaced by real-time, automated systems that give users visibility over their tax position throughout the year. Among the platforms gaining traction is Pie, a UK-based digital tax app built specifically to support self-employed individuals with modern income needs.
LONDON, United Kingdom - February 19, 2026 - Demand…
CivicMail.org Reinvents Postcard Campaigns for Grassroots Advocacy
CivicMail.org aims to bring civic engagement back to basics through the power of pen, paper, and postage.
Image: https://www.abnewswire.com/upload/2026/02/2addd1e9e0381d7e2262e1edbb064123.jpg
CivicMail.org [https://civicmail.org/] has announced its launch to help Americans send real, physical postcards to their elected officials with just a few clicks, delivering personalized messages directly to the desks of decision-makers at the local, state, and federal levels.
Research shows [https://www.concordia.ca/news/stories/2021/09/24/personalized-messages-are-more-likely-to-get-a-response-from-politicians-new-research-finds.html] that physical mail carries more weight with elected officials than petitions, emails, or…

New Children's Story: The Story of Sharin' Bear
A Heartfelt Message Of Courage, Kindness, And The True Meaning Of Giving
A pleasant new story for children, The Story of Sharin' Bear by Sharon Woods , introduces families to a lovable little cub whose journey of bravery and compassion changes him into a representation of sharing for children globally.
Entrenched in adventure, innocence, and emotional growth, this uplifting tale offers an unforgettable reminder that even the smallest acts of kindness can…
More Releases for Atopic
Rising Prevalence Of Atopic Dermatitis Is A Key Catalyst For The Growth Of The A …
Use code ONLINE20 to get 20% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
Atopic Dermatitis Market Size Valuation Forecast: What Will the Market Be Worth by 2025?
The scope of the atopic dermatitis market has experienced substantial expansion recently, projected to increase from $8.61 billion in 2024 to reach $9.88 billion in 2025, demonstrating a compound annual growth rate (CAGR) of 14.7%;…
Atopic Dermatitis Market Trends That Will Shape the Next Decade: Insights from P …
Stay ahead with our updated market reports featuring the latest on tariffs, trade flows, and supply chain transformations.
How Large Will the Atopic Dermatitis Market Size By 2025?
The market size for atopic dermatitis has seen significant expansion in the past few years. This expansion is anticipated to continue, with the market size increasing from $8.61 billion in 2024 to $9.96 billion in 2025, exhibiting a compound annual growth rate (CAGR) of…
Atopic Dermatitis Pipeline Outlook Report 2024
DelveInsight's, "Atopic Dermatitis Pipeline Insight 2024" report provides comprehensive insights about 100+ companies and 110+ pipeline drugs in the Atopic Dermatitis pipeline landscape. It covers the pipeline drug profiles, including Atopic Dermatitis clinical trials and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Key Takeaways from the Atopic Dermatitis Pipeline…
Advanced Therapy Revolutionizes Atopic Dermatitis Market Prospects
The atopic dermatitis market size is expected to be valued at USD 15.42 Billion in 2024 and reach USD 30.5 Billion by 2029, growing at a CAGR of 14.5 % from 2024 to 2029.
Atopic dermatitis, known as eczema, is a prevalent skin condition marked by red, inflamed, and itchy skin. It is a chronic condition commonly seen in children but can also impact adults. Atopic dermatitis is thought to result…
Atopic Dermatitis - Drug Pipeline Landscape, 2023
Atopic dermatitis (eczema) is a condition that causes dry, itchy and inflamed skin. It's characterized by inflamed skin that may crak and release a clear fluid when scratched. Eczema damages the skin barrier function that makes skin more sensitive and more prone to infection and dryness. Atopic dermatitis is caused by a combination of immune system activation, genetics, environmental triggers and stress. A weak skin barrier function might also trigger…
Atopic Dermatitis - Drug Pipeline Landscape, 2022
Atopic dermatitis (eczema) is a condition that causes dry, itchy and inflamed skin. It's characterized by inflamed skin that may cr*ck and release a clear fluid when scratched. Eczema damages the skin barrier function that makes skin more sensitive and more prone to infection and dryness.
Atopic dermatitis is caused by a combination of immune system activation, genetics, environmental triggers and stress. A weak skin barrier function might also trigger an…
