Press release
In-Vitro Diagnostics Market Outlook 2025-2032: Revenue to Surpass US$117.9 Bn
The global In-vitro Diagnostics (IVD) market is entering a new phase of technological maturity, driven by the convergence of molecular science, digital integration, and automation. According to market projections, the global IVD market size is estimated to be valued at US$ 77.4 billion in 2025, and is forecast to reach US$ 117.9 billion by 2032, reflecting a CAGR of 6.2% during the period 2025-2032.Between 2019 and 2024, the market recorded steady growth at a CAGR of 6.3%, highlighting consistent expansion. IVD technologies-comprising instruments, reagents, and analytical software-are revolutionizing diagnostic accuracy and efficiency. These tools enable the testing of biological samples such as blood, tissue, and saliva outside the human body, providing actionable clinical insights that underpin precision medicine, public health surveillance, and early disease detection.
Get a Sample Copy of Research Report (Use Corporate Mail id for Quick Response): https://www.persistencemarketresearch.com/samples/35529
The increasing emphasis on personalized healthcare, genomic profiling, and rapid diagnostic solutions continues to define market evolution. As healthcare systems shift toward preventive and value-based models, IVD has become indispensable in clinical decision-making.
Key Industry Highlights
Implementation of national screening programs for HPV, HIV, and hepatitis is bolstering test volumes across regions.
Rising direct-to-consumer (DTC) diagnostics is reshaping patient engagement and accessibility.
Growing collaboration between pharmaceutical and diagnostic firms is fostering advancements in companion diagnostics, particularly in immunotherapy and oncology.
Reagents are expected to hold around 66.4% of the market share in 2025, driven by the frequent need for replenishment and disease-specific assay kits.
Immunoassays are gaining traction for their high sensitivity and broad application in hormone, metabolic, and infectious disease testing.
Favorable reimbursement frameworks and regulatory clarity are accelerating molecular diagnostic adoption across North America and Europe.
Global Market Overview
Key Market Indicators Values
Market Size (2025E) US$ 77.4 Bn
Forecast Market Size (2032F) US$ 117.9 Bn
CAGR (2025-2032) 6.2%
Historical CAGR (2019-2024) 6.3%
Market Dynamics
1. Driver - Rising Burden of Chronic and Genetic Disorders
The global rise in chronic and genetic disorders such as Alzheimer's disease, Parkinson's disease, Turner syndrome, diabetes, and cardiovascular conditions is significantly boosting IVD adoption. Early diagnosis and ongoing monitoring through molecular and biochemical assays are vital in managing these diseases.
For instance, C2N Diagnostics' PrecivityAD2 blood test provides a non-invasive alternative for Alzheimer's screening by identifying amyloid plaques and tau proteins. Similarly, research into Parkinson's biomarkers is advancing IVD's role in neurological diagnostics. As chronic conditions demand continuous biomarker monitoring, the reliance on precise, scalable IVD platforms is growing rapidly.
2. Restraint - Pricing Barriers and Diagnostic Gaps
High costs of advanced molecular testing remain a critical restraint. Techniques such as digital PCR and next-generation sequencing (NGS) are often priced between US$ 3,000 and US$ 5,000 per test, limiting affordability in emerging markets.
The additional costs of equipment maintenance, validation, and skilled labor further elevate barriers to entry. Moreover, IVD's inability to fully capture the complexity of in vivo biological interactions can occasionally result in false negatives or limited diagnostic context. These factors hinder market penetration, particularly in low-resource regions where conventional testing remains dominant.
3. Opportunity - Expansion of Decentralized and Rapid Testing
The shift toward decentralized diagnostics and rapid point-of-care (PoC) solutions presents one of the most promising growth avenues. Portable and connected testing systems are transforming healthcare accessibility, especially in rural and emergency settings.
Abbott's ID NOW platform, offering molecular test results within 13 minutes, has exceeded 150 million global sales, underscoring the commercial potential of rapid diagnostics. Similarly, LumiraDx's microfluidic immunoassay platform provides multi-analyte testing in under 12 minutes. These innovations not only enhance patient convenience but also enable timely intervention in infectious disease outbreaks and chronic disease management.
Read Detailed Analysis: https://www.persistencemarketresearch.com/market-research/in-vitro-diagnostics-ivd-market.asp
Category-wise Analysis
1. Product Type Insights
The IVD market is segmented into instruments, reagents, and others, with reagents leading at approximately 66.4% share in 2025. Their recurrent use across molecular, immunoassay, and hematology applications ensures steady revenue streams.
Instrument demand is rising as laboratories adopt fully automated and modular systems that support high-throughput workflows and seamless integration with Laboratory Information Systems (LIS). Emerging markets are witnessing an influx of compact, affordable analyzers designed for decentralized operations in public health facilities.
2. Technology Insights
Key technologies in IVD include immunoassay, molecular diagnostics, clinical chemistry, hematology, coagulation, microbiology, and others.
Immunoassays are projected to hold 35.6% of the global share by 2025 due to their exceptional sensitivity and automation potential.
Molecular diagnostics remain the fastest-growing segment, driven by the surge in genetic and infectious disease testing.
Coagulation testing is gaining importance amid the increasing prevalence of cardiovascular and hematologic disorders such as deep vein thrombosis (DVT) and hemophilia.
Regional Insights
1. North America
North America is anticipated to command 48.2% of the global market share in 2025, with the United States leading due to its advanced healthcare infrastructure, robust R&D investments, and patient-centric diagnostics ecosystem.
The post-pandemic focus on early detection and personalized therapy has strengthened the adoption of molecular and companion diagnostics. Roche's Digital LightCycler System and Thermo Fisher Scientific's Ion Torrent Genexus platform exemplify the region's leadership in high-throughput, automated testing technologies.
2. Europe
Europe's IVD landscape is being reshaped by the implementation of the EU In Vitro Diagnostic Regulation (IVDR), replacing the former directive in 2022. The new regulatory framework emphasizes stringent quality, transparency, and safety compliance.
While smaller manufacturers face certification challenges, larger players are capitalizing on their established compliance infrastructure to consolidate market share. Germany, with its strong biomedical ecosystem, leads the region, followed by France, the UK, and the Nordics, which are investing heavily in diagnostic innovation and public health programs.
3. Asia Pacific
The Asia Pacific region is emerging as the fastest-growing IVD market, supported by expanding healthcare infrastructure, increasing healthcare expenditure, and local manufacturing capacity.
China is transitioning from import dependency to self-sufficiency by developing domestically manufactured reagents and molecular testing kits. India, through companies like Molbio Diagnostics, is revolutionizing diagnostics with platforms such as Truenat, offering portable PCR testing for diseases like tuberculosis, dengue, and HPV.
Government initiatives like the National Digital Health Mission (NDHM) are enhancing interoperability and driving digital integration between laboratories and healthcare providers. Additionally, Thailand, Malaysia, and Vietnam are seeing growth through medical tourism and public-private collaborations.
Request for Customization of the Research Report: https://www.persistencemarketresearch.com/request-customization/35529
Competitive Landscape
The IVD market is characterized by intense competition, continuous product innovation, and strategic alliances. Leading companies are expanding their molecular and PoC testing portfolios while forming partnerships with pharmaceutical firms to develop companion diagnostics for personalized therapies.
Major players include:
Abbott Laboratories
Siemens Healthineers AG
F. Hoffmann-La Roche Ltd.
Bio-Rad Laboratories, Inc.
Danaher Corporation
Becton, Dickinson and Company (BD)
bioMérieux SA
Agilent Technologies, Inc.
QIAGEN N.V.
Quest Diagnostics Incorporated
Quidel Corporation
These companies leverage strong distribution networks, R&D capabilities, and digital solutions to maintain competitive advantage in both developed and emerging markets.
Recent Developments
May 2025 - Diagnostics.ai launched the industry's first machine learning-based transparent PCR diagnostics platform-the CE-IVDR Strategic Advantage Platform, enhancing compliance with EU's new regulatory framework.
May 2025 - Nepal, in collaboration with WHO, introduced its National Essential In-vitro Diagnostics List (NEIDL), aimed at strengthening the country's diagnostic infrastructure under the Pandemic Fund initiative.
Ongoing partnerships between diagnostics and pharmaceutical firms are expected to expand the companion diagnostics market, particularly within oncology and rare disease segments.
Future Outlook
Looking ahead, the global IVD market is expected to experience sustained growth through 2032, propelled by breakthroughs in genomics, AI-driven diagnostics, and integrated digital health ecosystems.
The convergence of biotechnology, data analytics, and telemedicine will redefine diagnostic value chains, enhancing early disease detection and patient management. Emerging economies are projected to play a pivotal role in global expansion, driven by rising healthcare awareness, government funding, and local manufacturing initiatives.
By 2032, IVD is poised to become a cornerstone of precision healthcare, enabling clinicians to make faster, more informed decisions and improving outcomes across disease spectrums. The industry's ongoing transition toward affordable, portable, and data-integrated solutions positions it as a key enabler of the next generation of global healthcare delivery.
Contact Us:
Persistence Market Research
Second Floor, 150 Fleet Street, London, EC4A 2DQ, United Kingdom
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release In-Vitro Diagnostics Market Outlook 2025-2032: Revenue to Surpass US$117.9 Bn here
News-ID: 4235195 • Views: …
More Releases from Persistence Market Research

AI in Oncology Market Size to Surge from US$4.1Bn to US$18.3Bn by 2033: Persiste …
The advent of artificial intelligence (AI) has brought transformative changes across various industries, and healthcare is no exception. In oncology, the use of AI is revolutionizing the way cancer is diagnosed, treated, and managed, helping clinicians make faster, more accurate decisions. The AI in oncology market, which is currently valued at around US$ 4.1 billion in 2026, is projected to experience rapid growth, reaching an estimated US$ 18.3 billion by…

Overbed Table Market Valued at US$1.4 Bn in 2026, Says Persistence Market Resaer …
The global overbed table market is experiencing significant growth, fueled by a variety of factors, including an aging population, the increasing prevalence of chronic diseases, and the ongoing shift toward home-based healthcare solutions. With a forecasted market size of US$1.4 billion in 2026, the market is expected to reach US$2.1 billion by 2033, growing at a robust compound annual growth rate (CAGR) of 6.0% during the forecast period. This market…

3D Printed Prosthetics Market to Reach US$3.5Bn by 2033 at 8.3% CAGR - Persisten …
The 3D printed prosthetics market is poised for significant growth over the next decade, driven by advancements in additive manufacturing, materials innovation, and the increasing demand for personalized, cost-effective prosthetic solutions. The global market is expected to be valued at US$2.0 billion by 2026 and is projected to reach US$3.5 billion by 2033, growing at a compound annual growth rate (CAGR) of 8.3% during the forecast period from 2026 to…
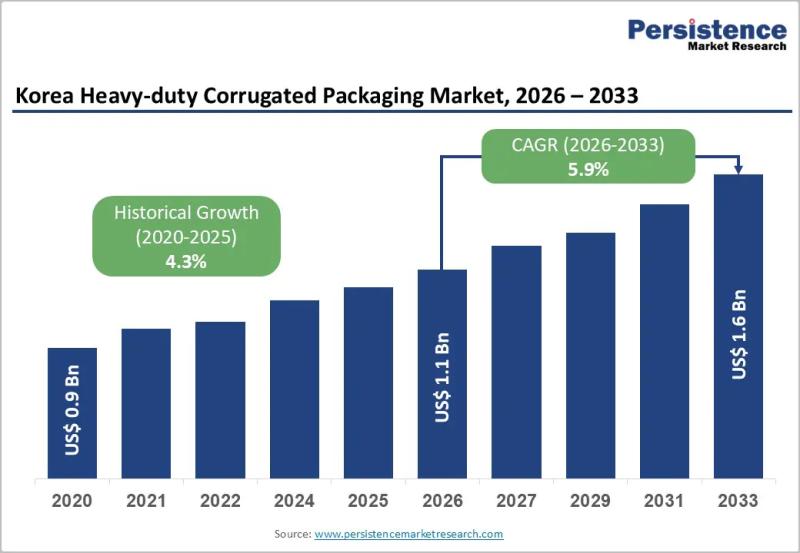
Korea Heavy-duty Corrugated Packaging Market to Reach US$1.6 Billion by 2033 - P …
The Korea heavy-duty corrugated packaging market plays a critical role in supporting industrial logistics, bulk transportation, and export-driven manufacturing. Heavy-duty corrugated packaging is widely used for shipping machinery, automotive components, electronics, chemicals, and large industrial goods that require superior strength and structural integrity. Unlike conventional corrugated boxes, heavy-duty variants are engineered with multi-wall boards, reinforced liners, and customized structural designs to withstand high load capacity, stacking pressure, and long-distance transportation.…
More Releases for IVD
Transformative Trends Impacting the Cancer In Vitro Diagnostics (IVD) Market Lan …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Cancer In Vitro Diagnostics (IVD) Market Size By 2025?
The market size for cancer in vitro diagnostics (IVD) has seen significant growth in the past few years. The market value, which is expected to be $13.36 billion in 2024, is projected to increase to $14.32…
In Vitro Diagnostics (IVD) Market
With the watchful use of established and advanced tools such as SWOT analysis and Porter's Five Forces Analysis, this market report has been structured. While preparing this In Vitro Diagnostics (IVD) Market research report, few of the attributes that have been adopted include highest level of spirit, practical solutions, committed research and analysis, innovation, integrated approaches, and most up-to-date technology.
Every possible effort has been taken while researching and analysing…
Companion Animal IVD Market - Guiding the Path to Optimal Health: Empowering Vet …
Newark, New Castle, USA - new report, titled Companion Animal IVD Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Companion Animal IVD market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Companion Animal IVD market. The report offers an overview of…
IVD Market 2021 | Detailed Report
The IVD market research report delivers accurate data and innovative corporate analysis, helping organizations of all sizes make appropriate decisions. The IVD report also incorporates the current and future global market outlook in the emerging and developed markets. Moreover, the report also investigates regions/countries expected to witness the fastest growth rates during the forecast period.
The IVD research report also provides insights of different regions that are contributing market growth.…
Liquid Biopsy IVD Market 2021 | Detailed Report
According to Market Study Report, Liquid Biopsy IVD Market provides a comprehensive analysis of the Liquid Biopsy IVD Market segments, including their dynamics, size, growth, regulatory requirements, competitive landscape, and emerging opportunities of global industry. An exclusive data offered in this report is collected by research and industry experts team.
Get Free Sample PDF (including full TOC, Tables and Figures) of Liquid Biopsy IVD Market @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=4623688
The report provides a…
Asia IVD Market
According to a new report published by Allied Market Research, the Asia Pacific In-vitro diagnostics market was valued at $12.9 billion in 2015, and is expected to reach $19.0 billion registering a CAGR of 5.6% during 2016 to 2022. The report offers a detailed analysis of the key segments, top investment pockets, changing dynamics, market size & estimations, and competitive scenario.
Download Free Sample Report @ https://www.alliedmarketresearch.com/request-sample/1256
The Asia-Pacific IVD market is…
