Press release
U.S. Preterm Birth Diagnostic Test Kit Market Growth Set at 6.3% CAGR Through 2032
The U.S. preterm birth diagnostic test kit market is projected to demonstrate steady growth in the coming years. The market size is likely to be valued at US $48.5 million in 2025 and is anticipated to reach US $74.4 million by 2032, expanding at a compound annual growth rate (CAGR) of 6.3% during the forecast period from 2025 to 2032. This growth reflects increasing awareness of preterm birth risks, rising adoption of diagnostic screening in maternal healthcare, and ongoing innovation in test technologies.Request a Sample: https://www.persistencemarketresearch.com/samples/35651
Growing Burden of Preterm Births in the U.S.
Preterm birth, defined as childbirth before 37 weeks of gestation, remains one of the leading causes of neonatal morbidity and mortality in the United States. Each year, an estimated 400,000 babies are born prematurely, posing significant medical and developmental challenges. These infants often face respiratory complications, neurological disorders, and other long-term health issues that place a heavy emotional and economic burden on families and healthcare systems.
The financial impact of preterm births is substantial, with healthcare costs related to neonatal intensive care and long-term support estimated at tens of billions of dollars annually. In response, medical professionals are increasingly focusing on preventive diagnostics to assess risk and intervene early. Diagnostic test kits play a crucial role in identifying women at high risk for preterm labor, enabling targeted treatment and monitoring to improve maternal and infant outcomes.
For More Information: https://www.persistencemarketresearch.com/market-research/us-preterm-birth-diagnostic-test-kit-market.asp
Key Market Drivers
Rising Awareness and Clinical Adoption
Growing awareness among clinicians and expectant mothers about the benefits of early detection is a major driver for market expansion. Hospitals, obstetric clinics, and diagnostic centers are incorporating advanced test kits as part of standard prenatal care, particularly for women presenting symptoms of preterm labor.
Dominance of Fetal Fibronectin (fFN) Testing
Among available diagnostic tools, Fetal Fibronectin (fFN) test kits hold the dominant market share. These tests have been clinically validated for decades and are approved for use in identifying women at risk of preterm birth. Their reliability and widespread clinical acceptance make them the preferred choice for many obstetric care providers.
Alternative test types, such as Placental Alpha Micro-Globulin-1 (PAMG-1) and Insulin-like Growth Factor Binding Protein-1 (iGFBP-1) assays, are gaining recognition but currently occupy smaller market segments due to limited penetration and cost considerations.
Shift Toward Point-of-Care (POC) Testing
A significant trend shaping the market is the shift from centralized laboratory testing toward point-of-care (POC) diagnostics. POC test kits provide rapid results, often within minutes, enabling real-time decision-making in hospital emergency departments and maternity wards. These tests reduce unnecessary admissions and improve clinical efficiency. As more POC technologies gain regulatory approval, their adoption is expected to accelerate, contributing significantly to overall market growth.
Integration into Value-Based Healthcare
The ongoing transformation of the U.S. healthcare system toward value-based care (VBC) models also supports the expansion of diagnostic testing. Under these frameworks, healthcare providers are incentivized to prevent complications rather than manage them after they occur. Preterm birth diagnostic kits that help reduce neonatal intensive care admissions and improve pregnancy outcomes align perfectly with these goals. Hospitals and insurers are recognizing the cost-effectiveness of early detection tools, which strengthens market demand.
Technological and Product Innovations
Emergence of Novel Biomarkers
Next-generation diagnostic technologies are expanding beyond traditional biomarkers. Research in blood-based diagnostics, cell-free RNA (cfRNA) profiling, and proteomics is enabling earlier, more accurate prediction of preterm birth risk. These advanced assays can identify biochemical changes long before symptoms appear, opening the door for preventive interventions.
The introduction of blood-based predictive tests, such as the PreTRM® test, which analyzes maternal proteins during mid-pregnancy to estimate preterm risk, exemplifies how innovation is reshaping the market. Similar technologies are under development by several biotechnology and diagnostics firms, aiming to provide non-invasive, high-sensitivity solutions.
Multiplex and AI-Enabled Testing
Future diagnostic kits are likely to combine multiple biomarkers in a single assay and integrate artificial intelligence (AI) for data interpretation. Such hybrid approaches promise improved accuracy and predictive power. The integration of digital health tools for tracking and interpreting results also enhances accessibility, particularly in remote or underserved regions.
Do You Have Any Query or Specific Requirement? Request Customization of Report:
https://www.persistencemarketresearch.com/request-customization/35651
Market Segmentation Insights
By Product Type
• Fetal Fibronectin (fFN) Test Kits: Leading the market with extensive clinical validation and strong reimbursement support.
• PAMG-1 and iGFBP-1 Test Kits: Emerging as alternatives with potential for wider adoption as cost efficiency and ease of use improve.
• Multiplex Panels: Under development, expected to offer comprehensive risk assessment by combining several biomarkers in one test.
By Sample Type
• Vaginal Discharge (Cervicovaginal Fluid): Dominates the market due to well-established collection methods and strong diagnostic accuracy.
• Blood Samples: Gaining momentum as non-invasive options for earlier prediction.
• Urine Samples: Represent a smaller but evolving segment, supported by convenience and patient comfort.
By Technology
• Point-of-Care Testing: Fastest-growing segment due to immediate results and suitability for hospital or outpatient settings.
• Laboratory-Based Testing: Continues to serve as the standard for confirmatory and high-throughput applications.
By End User
• Hospitals: Hold the largest market share due to high patient volume, diagnostic infrastructure, and reimbursement availability.
• Outpatient Clinics and Diagnostic Centers: Increasing adoption of compact, easy-to-use kits for quick screening.
• Home and Telehealth Settings: Expected to gain traction as self-testing and remote monitoring technologies mature.
Competitive Landscape
The U.S. preterm birth diagnostic test kit market is moderately consolidated, featuring both established diagnostics companies and emerging innovators. Key industry participants include:
• Hologic, Inc.
• Sera Prognostics
• QIAGEN Sciences LLC
• Actim Oy
• Creative Diagnostics
• Laborie
• Elabscience Bionovation Inc.
• CUSABIO Technology LLC
• Biosynex SA
• IQ Products
These players are actively investing in research and development, clinical validation, and product diversification to strengthen their market positions.
Recent strategic activities include product launches, regulatory approvals, and collaborations with healthcare institutions. For example, new blood-based and at-home test solutions have entered clinical evaluation phases, reflecting the industry's drive toward patient-centric innovation. Companies are also integrating digital tools and AI to improve accuracy, streamline workflows, and enhance diagnostic confidence.
Recent Developments
Several key developments have occurred in the U.S. preterm birth diagnostic test landscape:
• In 2025, a leading maternal-health company introduced a next-generation at-home blood test leveraging RNA-based biomarkers to assess preterm birth risk with high predictive accuracy.
• In 2024, clinical trials such as the AVERT PRETERM Study demonstrated measurable reductions in neonatal morbidity and mortality through test-and-treat approaches using validated diagnostic tools.
• Diagnostic startups and medical device companies are expanding their pipelines through partnerships with hospitals and research institutions to validate new predictive biomarkers.
Such advancements are expected to accelerate the transition from symptom-based testing to preventive, population-scale screening.
Challenges and Restraints
Despite promising growth, the market faces a few challenges that could limit rapid expansion:
1. Variability in Test Performance: Diagnostic accuracy can differ depending on sample quality, timing of testing, and patient conditions.
2. Reimbursement Barriers: Limited or inconsistent insurance coverage can hinder adoption, particularly among smaller healthcare providers.
3. Regulatory Hurdles: New biomarkers require extensive clinical validation and approval processes before commercialization.
4. Cost Sensitivity: High test prices or lack of bundled reimbursement may slow adoption in resource-constrained settings.
5. Competition from Alternative Tools: Traditional clinical assessments, ultrasound, and monitoring methods may still be preferred in certain healthcare facilities.
Addressing these challenges will require strong clinical evidence, regulatory clarity, payer engagement, and continued cost optimization.
Future Outlook
The outlook for the U.S. preterm birth diagnostic test kit market remains positive. The industry is evolving toward non-invasive, early-stage screening solutions that integrate seamlessly into standard prenatal care. Key trends expected to shape the market over the next decade include:
• Wider Adoption of Blood-Based Testing: Offering earlier and more convenient screening opportunities.
• Expansion of At-Home Diagnostics: Allowing pregnant women to monitor risk remotely, improving access to care.
• AI-Powered Predictive Analytics: Combining biomarkers with patient data for more personalized and accurate risk assessments.
• Integration into Digital Health Platforms: Enabling real-time data sharing and telehealth consultation for expectant mothers.
• Greater Focus on Cost Efficiency: As healthcare systems shift toward value-based care, affordable and validated diagnostics will see higher adoption rates.
With ongoing research and increasing healthcare emphasis on maternal and neonatal well-being, the market is well-positioned for sustained expansion through 2032.
Conclusion
The U.S. preterm birth diagnostic test kit market is set for notable growth between 2025 and 2032, driven by rising awareness, technological advancements, and integration into modern maternal care. Valued at US $48.5 million in 2025, the market is projected to reach US $74.4 million by 2032, expanding at a CAGR of 6.3%.
As the demand for early, reliable, and cost-effective testing continues to grow, diagnostic companies are focusing on developing next-generation biomarkers, AI-driven analytics, and user-friendly POC devices. These innovations are expected to transform prenatal care, reduce the rate of premature births, and enhance healthcare outcomes for both mothers and infants.
Explore the Latest Trending Research Reports:
U.S. Surgical Microscope Market -https://www.persistencemarketresearch.com/market-research/us-surgical-microscope-market.asp
High-Flow-Oxygen-Therapy Devices Market -https://www.persistencemarketresearch.com/market-research/high-flow-oxygen-therapy-devices-market.asp
U.S. Intraoral Scanners Market -https://www.persistencemarketresearch.com/market-research/us-intraoral-scanners-market.asp
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
Contact Us:
Persistence Market Research
Second Floor, 150 Fleet Street,
London, EC4A 2DQ, United Kingdom
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release U.S. Preterm Birth Diagnostic Test Kit Market Growth Set at 6.3% CAGR Through 2032 here
News-ID: 4228171 • Views: …
More Releases from Persistence Market Research

Rolling Papers Market Projected to Reach US$2.5 Billion by 2033, Growing at a CA …
The global rolling papers market is experiencing steady growth, fueled by the increasing demand for both tobacco and cannabis-related products. According to the latest study by Persistence Market Research, the market size is expected to be valued at US$1.8 billion in 2026 and is projected to reach US$2.5 billion by 2033, growing at a compound annual growth rate (CAGR) of 4.8% during the forecast period. This growth is attributed to…

Critical Power and Cooling Market Projected to Reach US$ 53.6 Billion by 2033, G …
The global critical power and cooling market is experiencing significant growth, driven by the increasing demand for uninterrupted power supply and cooling systems across various industries. According to the latest study by Persistence Market Research, the market size is expected to be valued at US$ 31.9 billion in 2026 and is projected to reach US$ 53.6 billion by 2033, growing at a compound annual growth rate (CAGR) of 7.7% during…

Self-Healing Concrete Market Projected Growth to US$463.6 Billion by 2033 - Pers …
The global self-healing concrete market is poised for rapid expansion in the coming years, with the latest study by Persistence Market Research forecasting its value to grow from US$106.4 billion in 2026 to US$463.6 billion by 2033, reflecting a compound annual growth rate (CAGR) of 23.4% during the forecast period from 2026 to 2033. This remarkable growth is driven by increasing demand for durable and sustainable construction materials, propelled by…
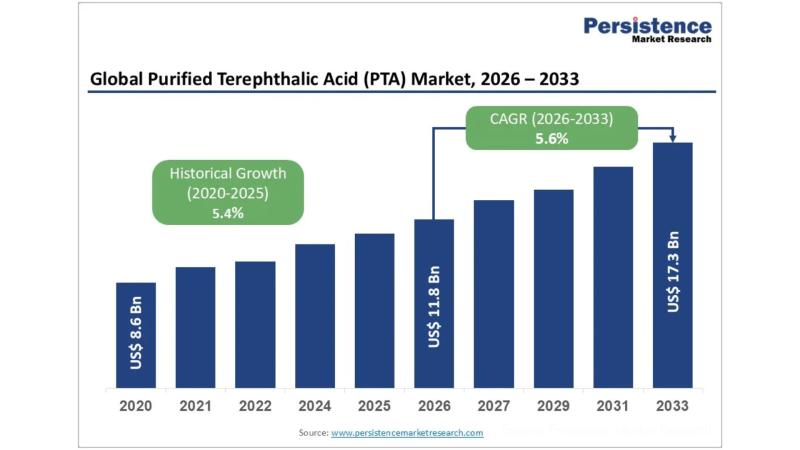
Purified Terephthalic Acid (PTA) Market Growth and Trends: Projected to Reach $1 …
The global purified terephthalic acid (PTA) market is poised for substantial growth in the coming years. According to the latest study by Persistence Market Research, the market is projected to expand from a valuation of US$11.8 billion in 2026 to US$17.3 billion by 2033, reflecting a compound annual growth rate (CAGR) of 5.6% during the forecast period from 2026 to 2033. The growth is primarily driven by the increasing demand…
More Releases for Test
Key Differences Between Megger Test, Tan Delta Test, and Hi-Pot Test for Electri …
Electrical insulation plays a critical role in ensuring the safety and efficiency of electrical systems. To assess the condition of insulation and identify potential issues, three common tests are used: the Megger test, Tan Delta test, and Hi-Pot test. Each test serves a unique purpose and provides valuable insights into the state of electrical insulation. Here's a closer look at the differences between these three essential tests.
Megger Test: Insulation Resistance…
Vitamin Test Market: Global Vitamin Test Analysis and Forecast (2023-2029)Vitami …
12.04.2024: Vitamin Test Market Overview
The development of companion diagnostic tools and advances in personalised treatment are driving considerable growth and revolution in the oncology Vitamin Test market. In the era of precision medicine, where healthcare is increasingly customised for individual individuals based on their own genetic and molecular profiles, this market segment is essential. Ongoing innovation and development define the oncology Vitamin Test market. To find particular biomarkers, genetic mutations,…
CAGR 8.1% Homecare Pregnancy Test Kits Market By Type of Test (Urine Test For H …
The Homecare Pregnancy Test Kits market report by Reports and Data provides an extensive overview of the vital elements of the Homecare Pregnancy Test Kits market and factors such as the drivers, restraints, latest trends, supervisory scenario, competitive landscape, technological advancements, and others. Further, it mentions the market shares associated with the market in terms of both value and volume along with the segmentation. Space-age industrial and digitalization tools are…
Home Safety Test Kits Market, Home Safety Test Kits Market Trends, Home Safety T …
“Home Safety Test Kits Market” 2020-2025 Research Report is a professional and in-depth study on the current state of the market. Global Home Safety Test Kits market containing a complete view of the market size, business share, profit estimates, SWOT analysis and the regional landscape of the Industry. The report explains key challenges and future development prospects of the market. The Global Home Safety Test Kits analysis is provided for…
Test Data Management (TDM) Market - test data profiling, test data planning, tes …
The report categorizes the global Test Data Management (TDM) market by top players/brands, region, type, end user, market status, competition landscape, market share, growth rate, future trends, market drivers, opportunities and challenges, sales channels and distributors.
This report studies the global market size of Test Data Management (TDM) in key regions like North America, Europe, Asia Pacific, Central & South America and Middle East & Africa, focuses on the consumption…
Hearing Screening and Diagnostic Devices Market Demands with Major Tests: pure T …
New Market Research Reports Title "Hearing Screening And Diagnostic Devices Market 2018" Has Been Added to Crystal Market Research Report database.
Hearing Screening and Diagnostic Devices - Competitive Insights:
The leading players in the market are Gn Otometrics A/S, Otodynamics, Nashua Hearing Group, Siemens Healthineers, Natus Medical Incorporated, Interacoustics A/S, Neurosoft S.A, Accent Hearing Pty Ltd, MAICO Diagnostics GmbH and IntriCon Corporation. The major players in the market are profiled in detail…
