Press release
Presbyopia Pipeline 2025: Innovative Clinical Developments by Leading Global Companies, Featuring Visus Therapeutics, Glaukos Corporation, Cellix Bio, Orasis Pharmaceuticals, Cellix Bio
DelveInsight's, "Presbyopia - Pipeline Insight, 2025" report provides comprehensive insights about 6+ companies and 6+ pipeline drugs in Presbyopia pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.As presbyopia becomes increasingly prevalent worldwide and is associated with comorbidities such as diabetes, cardiovascular disease, and certain cancers, the demand for safer and more effective treatments is rising. According to DelveInsight, the presbyopia pipeline includes over ten pharmaceutical and biotech companies actively developing more than ten therapeutic candidates. These treatments are at various stages of clinical and preclinical development, highlighting significant innovation and commitment to addressing this widespread public health challenge.
DelveInsight's "Presbyopia Pipeline Insight 2025" report offers a comprehensive and strategic analysis of the current R&D landscape. It provides details on clinical trial progress, emerging therapies, mechanisms of action, competitive positioning, and key initiatives from leading companies such as Visus Therapeutics, Glaukos Corporation, Cellix Bio, and Orasis Pharmaceuticals. The report serves as an essential resource for researchers, healthcare investors, and decision-makers seeking insights into the evolving presbyopia therapeutics market and the breakthroughs shaping its future.
Explore the Cutting-Edge Landscape of Presbyopia Drug Development @ https://www.delveinsight.com/report-store/presbyopia-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
Key Takeaways from the Presbyopia Pipeline Report
*
DelveInsight's report on the presbyopia pipeline highlights a dynamic landscape, with over ten companies actively developing more than ten therapeutic candidates for presbyopia treatment.
*
In July 2025, LENZ partnered with Laboratoires Thea to commercialize LNZ100 in Canada. Its U.S. NDA has been accepted by the FDA, with a target decision date of August 8, 2025. In June 2025, the FDA accepted Tenpoint Therapeutics' NDA for BRIMOCHOL Trademark PF, a fixed-dose combination eye drop (carbachol + brimonidine), with a PDUFA date set for January 28, 2026, and no advisory committee planned.
*
Leading companies in the presbyopia space-including Visus Therapeutics, Glaukos Corporation, Cellix Bio, Orasis Pharmaceuticals, and others-are actively exploring new therapies to enhance the treatment landscape. Promising pipeline candidates in various stages of development include LNZ100, GLK-302, and additional investigational treatments.
Presbyopia Overview:
Presbyopia is an age-related vision condition characterized by the gradual decline in the eye's ability to focus on nearby objects, caused by reduced lens flexibility. It typically begins around age 40 and represents one of the most common physiological changes in adults. Symptoms include eye strain, headaches, blurred near vision, and the need to hold reading materials at a greater distance. Extended screen use can exacerbate issues such as delayed focusing and visual fatigue. Reading glasses remain the standard treatment. Although the precise mechanism is still debated, increased lens stiffness is widely accepted as the primary cause. The prevalence of presbyopia in low- and middle-income countries is likely underreported, as most research emphasizes distance vision.
Download the Presbyopia sample report to know in detail about the Presbyopia treatment market [https://www.delveinsight.com/sample-request/presbyopia-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Presbyopia Pipeline Analysis
The Presbyopia pipeline insights report 2025, provides insights into:
*
Provides comprehensive insights into key companies developing therapies in the Presbyopia Market.
*
Categorizes Presbyopia therapeutic companies by development stage: early, mid, and late-stage.
*
Highlights major companies involved in targeted therapy development, including both active and inactive (paused/discontinued) projects.
*
Reviews emerging Presbyopia drugs under development based on:
*
Stage of development
*
Presbyopia Route of administration
*
Target receptor
*
Monotherapy vs. combination therapy
*
Presbyopia Mechanism of action
*
Molecular type
*
Offers detailed analysis of:
*
Company-to-company and company-academia collaborations
*
Presbyopia Licensing agreements
*
Funding and investment activities supporting future Presbyopia market advancement.
Unlock key insights into emerging Presbyopia therapies and market strategies here: https://www.delveinsight.com/report-store/presbyopia-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
Presbyopia Emerging Drugs
*
LNZ100: Lenz Therapeutics
LNZ100 is a preservative-free, once-daily eye drop containing aceclidine, a selective muscarinic receptor agonist. It enhances near vision by constricting the pupil, producing a pinhole effect. Unlike other miotic agents, aceclidine minimizes ciliary muscle stimulation, reducing side effects such as brow ache and myopic shift. This makes LNZ100 a well-tolerated, long-acting treatment suitable for a broad range of presbyopia patients, a condition caused by age-related lens stiffening. The FDA has accepted LNZ100's New Drug Application (NDA), and the drug is currently in the registration phase for presbyopia therapy.
*
GLK-302: Glaukos Corporation
Glaukos Corporation is developing GLK-302, a sterile topical ophthalmic cream containing pilocarpine for the treatment of presbyopia. Applied to the eyelid, the cream allows pilocarpine to penetrate the skin and reach the eye. As a muscarinic acetylcholine receptor agonist, pilocarpine acts on M1 and M3 receptors, inducing pupillary constriction and increasing depth of focus. This mechanism enhances near vision in presbyopic patients without substantially affecting distance vision. GLK-302 is currently in Phase II clinical trials for presbyopia.
Presbyopia Pipeline Therapeutic Assessment
Presbyopia Assessment by Product Type
- Mono
- Combination
- Mono/Combination
Presbyopia By Stage
- Late-stage products (Phase III)
- Mid-stage products (Phase II)
- Early-stage product (Phase I) along with the details of
- Pre-clinical and Discovery stage candidates
- Discontinued & Inactive candidates
Presbyopia Assessment by Route of Administration
- Oral
- Parenteral
- Intravenous
- Subcutaneous
- Topical
Presbyopia Assessment by Molecule Type
- Recombinant fusion proteins
- Small molecule
- Monoclonal antibody
- Peptide
- Polymer
- Gene therapy
Download sample pages to get an in-depth assessment of the emerging Presbyopia therapies and key Presbyopia companies [https://www.delveinsight.com/sample-request/presbyopia-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Contents
1. Report Introduction
2. Executive Summary
3. Presbyopia Current Treatment Patterns
4. Presbyopia - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Presbyopia Late-Stage Products (Phase-III)
7. Presbyopia Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Presbyopia Discontinued Products
13. Presbyopia Product Profiles
14. Presbyopia Key Companies
15. Presbyopia Key Products
16. Dormant and Discontinued Products
17. Presbyopia Unmet Needs
18. Presbyopia Future Perspectives
19. Presbyopia Analyst Review
20. Appendix
21. Report Methodology
Request the sample PDF to get detailed insights about the Presbyopia pipeline reports offerings: https://www.delveinsight.com/report-store/presbyopia-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=presbyopia-pipeline-2025-innovative-clinical-developments-by-leading-global-companies-featuring-visus-therapeutics-glaukos-corporation-cellix-bio-orasis-pharmaceuticals-cellix-bio]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Presbyopia Pipeline 2025: Innovative Clinical Developments by Leading Global Companies, Featuring Visus Therapeutics, Glaukos Corporation, Cellix Bio, Orasis Pharmaceuticals, Cellix Bio here
News-ID: 4227967 • Views: …
More Releases from ABNewswire
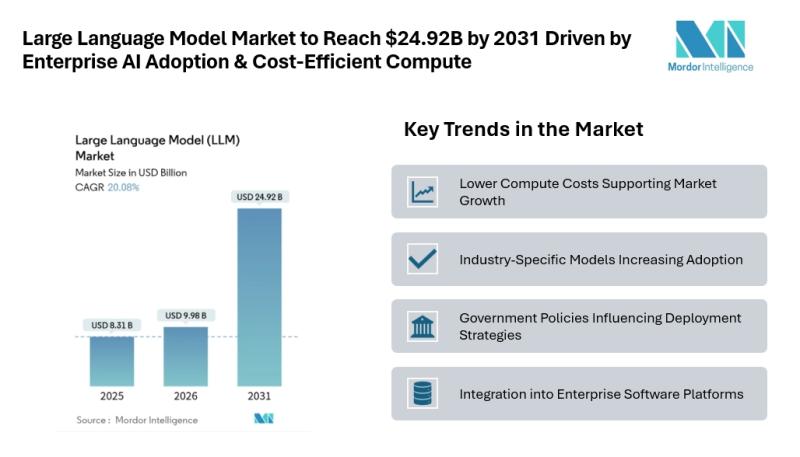
Large Language Model Market to Reach $24.92B by 2031 Driven by Enterprise AI Ado …
Mordor Intelligence has published a new report on the large language model market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Large Language Model Market Outlook
According to Mordor Intelligence, the LLM market size [https://www.mordorintelligence.com/industry-reports/large-language-model-llm-market?utm_source=abnewswire] was valued at USD 8.31 billion in 2025 and is estimated to grow to USD 9.98 billion in 2026, reaching USD 24.92 billion by 2031 at a CAGR of 20.08% during the forecast period.…

Self Employed Tax Software UK: Why Freelancers and Sole Traders Are Switching to …
With Many individuals are seeking software that simplifies tax filing while ensuring full compliance with HMRC requirements. Manual spreadsheets and paper-based calculations are being replaced by real-time, automated systems that give users visibility over their tax position throughout the year. Among the platforms gaining traction is Pie, a UK-based digital tax app built specifically to support self-employed individuals with modern income needs.
LONDON, United Kingdom - February 19, 2026 - Demand…
CivicMail.org Reinvents Postcard Campaigns for Grassroots Advocacy
CivicMail.org aims to bring civic engagement back to basics through the power of pen, paper, and postage.
Image: https://www.abnewswire.com/upload/2026/02/2addd1e9e0381d7e2262e1edbb064123.jpg
CivicMail.org [https://civicmail.org/] has announced its launch to help Americans send real, physical postcards to their elected officials with just a few clicks, delivering personalized messages directly to the desks of decision-makers at the local, state, and federal levels.
Research shows [https://www.concordia.ca/news/stories/2021/09/24/personalized-messages-are-more-likely-to-get-a-response-from-politicians-new-research-finds.html] that physical mail carries more weight with elected officials than petitions, emails, or…

New Children's Story: The Story of Sharin' Bear
A Heartfelt Message Of Courage, Kindness, And The True Meaning Of Giving
A pleasant new story for children, The Story of Sharin' Bear by Sharon Woods , introduces families to a lovable little cub whose journey of bravery and compassion changes him into a representation of sharing for children globally.
Entrenched in adventure, innocence, and emotional growth, this uplifting tale offers an unforgettable reminder that even the smallest acts of kindness can…
More Releases for Presbyopia
Presbyopia Pipeline Assessment Report 2025 | DelveInsight
DelveInsight's, "Presbyopia Pipeline Insight, 2025" report provides comprehensive insights about 10+ companies and 10+ pipeline drugs in Presbyopia pipeline landscape. It covers the Presbyopia pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Presbyopia pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Discover the latest drugs and treatment options in the Presbyopia…
Rising Geriatric Population Fuels Growth Of Myopia And Presbyopia Treatment Mark …
The Myopia and Presbyopia Treatment Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Myopia and Presbyopia Treatment Market Size and Projected Growth Rate?
The market size for myopia and presbyopia treatment has witnessed substantial growth over several recent years. The market is projected…
Myopia and Presbyopia Treatment Market: Addressing Vision Impairment Globally
The global market for myopia and presbyopia treatment has been witnessing remarkable growth, driven by the rising prevalence of vision impairment worldwide. As per recent findings from Transparency Market Research, the market was valued at approximately US$16.7 billion in 2021 and is projected to reach nearly US$45 billion by 2032, with a promising CAGR of 9.5% over the next decade. Corrective lenses emerge as the leading treatment type, with revenue…
Presbyopia Treatment Market Growth Analysis 2023-2030
Global Presbyopia Treatment Market Gains Momentum Amidst Rising Aging Population and Innovations in Therapeutics:
The global presbyopia treatment market has witnessed substantial growth in recent years, fueled by the increasing prevalence of presbyopia and the expanding aging population. Presbyopia, a common vision loss associated with aging, has prompted a surge in demand for effective treatments, leading to advancements in eyeglasses, contact lenses, surgeries, eye drops, and nutrient-based interventions.
Refractive surgeries, including Conductive…
Presbyopia Pipeline: Insights into Novel Pipeline Therapies, Key Pharma Companie …
DelveInsight's, "Presbyopia Pipeline Insight, 2022," report provides comprehensive insights about 12+ companies and 12+ pipeline drugs in Presbyopia pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Key takeaways from the Presbyopia Pipeline Insight Report
• Presbyopia Pipeline report offers a comprehensive…
Presbyopia Market 2020-2027 Sets the Table for Continued Growth || Top Growing C …
A transparent research method has been accomplished with the right tools and techniques to make this Presbyopia Market research report world-class. Two of the most widely used techniques namely SWOT analysis and Porter's Five Forces Analysis have been used while generating this report. Competitive analysis conducted in this report puts light on the moves of the key players in the Healthcare industry such as new product launches, expansions, agreements, joint…
