Press release
Australia Biosimilar Market Projected to Reach USD 5,512.5 Million by 2033
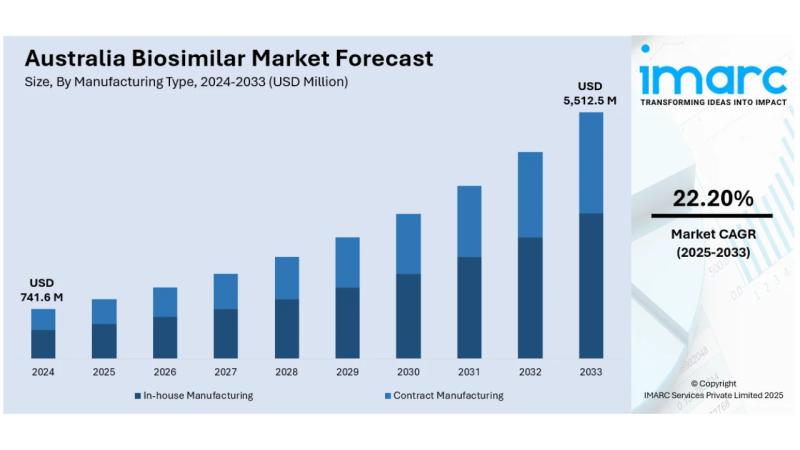
The latest report by IMARC Group, titled "Australia Biosimilar Market Report by Molecule (Infliximab, Insulin Glargine, Epoetin Alfa, Etanercept, Filgrastim, Somatropin, Rituximab, Follitropin Alfa, Adalimumab, Pegfilgrastim, Trastuzumab, Bevacizumab, Others), Manufacturing Type (In-house Manufacturing, Contract Manufacturing), Indication (Auto-Immune Diseases, Blood Disorder, Diabetes, Oncology, Growth Deficiency, Female Infertility, Others), and Region 2025-2033," offers a comprehensive analysis of the Australia biosimilar market growth. The report includes competitor and regional analysis, along with a detailed breakdown of the market segmentation. The Australia biosimilar market size reached USD 741.6 Million in 2024. Looking forward, IMARC Group expects the market to reach USD 5,512.5 Million by 2033, exhibiting a CAGR of 22.20% during 2025-2033.Base Year: 2024
Forecast Years: 2025-2033
Historical Years: 2019-2024
Market Size in 2024: USD 741.6 Million
Market Forecast in 2033: USD 5,512.5 Million
Market Growth Rate (2025-2033): 22.20%
Australia Biosimilar Market Overview
The Australia biosimilar market is experiencing explosive growth driven by supportive government policies including PBS listings and PBAC recommendations, rising demand for affordable biologic treatments addressing chronic diseases, increasing clinical trial activity enhancing market credibility, broader acceptance among healthcare providers supported by regulatory clarity, patent expiry of high-cost biologics creating competitive opportunities, and rising chronic disease burden requiring long-term biologic therapies. The market expansion is supported by private sector investment in development and commercialization, Pharmaceutical Benefits Scheme subsidy enablement, pharmacy-level substitution policies, government prescribing practice encouragement programs, and growing physician confidence through educational initiatives. Enhanced regulatory approval pathways, therapeutic equivalence demonstration, and cost-effectiveness positioning are positioning Australia's biosimilar market for sustained growth and healthcare affordability leadership.
Australia's biosimilar industry demonstrates strong regulatory foundation through Therapeutic Goods Administration oversight and Pharmaceutical Benefits Advisory Committee evaluation ensuring quality, safety, and efficacy standards comparable to originator biologics. The market maintains critical importance in managing chronic conditions including oncology, rheumatology, gastroenterology, autoimmune diseases, and diabetes with biosimilars offering comparable therapeutic benefits at significantly reduced costs. The proliferation of patent expirations, clinical research programs establishing local expertise, international manufacturer interest, and hospital tender-based procurement is creating favorable market conditions, requiring substantial investments in manufacturing capabilities, physician education, patient awareness campaigns, and distribution infrastructure. Australia's strategic focus on healthcare sustainability and treatment accessibility, combined with world-class clinical research environment and supportive reimbursement frameworks, makes it an increasingly dynamic market for biosimilar innovation and affordable biologic therapy provision.
Request For Sample Report:
https://www.imarcgroup.com/australia-biosimilar-market/requestsample
Australia Biosimilar Market Trends
• PBS listing acceleration: Significant government support through Pharmaceutical Benefits Scheme integration with PBAC recommending products like Amgen's Wezlana (ustekinumab biosimilar) in May 2024 following TGA approval signaling biosimilar acceptance into frontline treatment plans.
• Clinical research leadership: Strong clinical trial infrastructure attracting international developers with Phase I study of SELARSDI (ustekinumab-taken) by Alvotech and Teva in April 2024 supporting FDA approval and reinforcing Australia's global biosimilar development standing.
• Healthcare provider acceptance growth: Increasing physician confidence driven by substantial clinical data, favorable real-world outcomes, and clear TGA regulatory guidance encouraging switching from originator biologics particularly in oncology, rheumatology, and gastroenterology.
• Patent cliff opportunities: Major biologic patent expirations creating competitive landscape enabling both local and international manufacturers to launch biosimilars targeting cancer, rheumatoid arthritis, diabetes, and other chronic conditions.
• Chronic disease treatment focus: Rising prevalence of cancer, autoimmune diseases, and diabetes requiring long-term biologic therapies with biosimilars providing comparable safety and efficacy at lower prices enabling scalable healthcare solutions.
• Private investment surge: Heightened pharmaceutical company interest in biosimilar development and commercialization driven by favorable regulatory conditions, increasing affordable treatment demand, and developing healthcare infrastructure creating market opportunities.
Market Drivers
• Government policy support: Comprehensive regulatory backing through PBS listings, PBAC evaluations, and streamlined TGA approval pathways ensuring patients receive cost-effective treatments while encouraging biosimilar developer competition and market entry.
• Healthcare cost containment: Public and private healthcare providers seeking biosimilars to manage long-term treatment costs while ensuring broad accessibility addressing healthcare budget pressures and improving treatment sustainability.
• Clinical evidence accumulation: Growing body of clinical trial data, real-world outcomes, and post-marketing surveillance building physician confidence and demonstrating biosimilar therapeutic equivalence to originator biologics across multiple indications.
• Chronic disease epidemiology: Increasing incidence of cancer, rheumatoid arthritis, inflammatory bowel disease, and diabetes creating sustained demand for affordable long-term biologic therapies supporting biosimilar market expansion.
• Regulatory clarity advancement: Clear Therapeutic Goods Administration guidelines, interchangeability frameworks, and substitution policies reducing uncertainty and facilitating faster market entry for biosimilar manufacturers seeking Australian approval.
• Hospital procurement initiatives: Public tender-based purchasing enabling government and hospital networks to acquire biosimilars at competitive rates expanding patient access while securing stable revenue streams for manufacturers.
Challenges and Opportunities
Challenges:
• Physician reluctance with some healthcare providers hesitant to switch stable patients from originator biologics due to concerns about immunogenic responses and varying clinical outcomes particularly in complex oncology and autoimmune treatments
• Limited patient awareness with many individuals unfamiliar with biosimilars mistakenly equating them with generic drugs or lacking understanding of quality and effectiveness comparisons to originator biologics creating switching skepticism
• Originator brand loyalty with established biologics maintaining market dominance through strong marketing strategies, physician preference cultivation, and patient trust requiring biosimilar manufacturers to overcome entrenched market positions
• Substitution barriers with regulatory restrictions and physician approval requirements for biosimilar switching creating administrative hurdles and limiting pharmacy-level dispensing flexibility in certain therapeutic areas
• Manufacturing complexity with biosimilar production requiring sophisticated biologic manufacturing capabilities, quality control systems, and regulatory compliance expertise creating high entry barriers and capital investment requirements
Opportunities:
• Oncology and autoimmune therapy expansion targeting high-cost treatment areas with long-term therapy requirements where biosimilars can bridge affordability gaps while maintaining efficacy across breast cancer, rheumatoid arthritis, and inflammatory conditions
• Hospital tender participation securing long-term institutional contracts through public procurement in high-demand areas including oncology, nephrology, and endocrinology providing stable revenue streams and market penetration advantages
• Local manufacturing development establishing domestic biomanufacturing facilities offering quicker delivery times, better regulatory responsiveness, reduced international logistics dependence, and alignment with healthcare sovereignty goals
• Digital health integration leveraging telehealth platforms, online patient education, and electronic prescribing systems to increase biosimilar awareness, simplify switching processes, and enhance accessibility across regional areas
• Physician education partnerships implementing continuing medical education programs, clinical evidence presentations, and switching protocol guidance building prescriber confidence and normalizing biosimilar integration into standard treatment practices
Australia Biosimilar Market Segmentation
By Molecule:
• Infliximab
• Insulin Glargine
• Epoetin Alfa
• Etanercept
• Filgrastim
• Somatropin
• Rituximab
• Follitropin Alfa
• Adalimumab
• Pegfilgrastim
• Trastuzumab
• Bevacizumab
• Others
By Manufacturing Type:
• In-house Manufacturing
• Contract Manufacturing
By Indication:
• Auto-Immune Diseases
• Blood Disorder
• Diabetes
• Oncology
• Growth Deficiency
• Female Infertility
• Others
By Region:
• Australia Capital Territory & New South Wales
• Victoria & Tasmania
• Queensland
• Northern Territory & Southern Australia
• Western Australia
Browse Full Report:
https://www.imarcgroup.com/australia-biosimilar-market
Australia Biosimilar Market News (2024-2025)
• May 2024: Pharmaceutical Benefits Advisory Committee (PBAC) recommended Amgen's Wezlana, first ustekinumab biosimilar, for PBS listing following January 2024 TGA approval for Crohn's disease, ulcerative colitis, plaque psoriasis, and psoriatic arthritis indications.
• April 2024: Alvotech and Teva conducted Phase I study of SELARSDI (ustekinumab-taken) in Australia and New Zealand playing pivotal role in securing US FDA approval and strengthening Australia's global standing in biosimilar development.
• 2024: Healthcare provider acceptance expanded with physicians increasingly confident in biosimilar safety and effectiveness driven by substantial clinical data, real-world outcomes, and clear Therapeutic Goods Administration regulatory guidance.
• 2024: Private sector investment intensified with pharmaceutical companies expanding local operations, forming partnerships, and launching new biosimilar products to capture early market opportunities in favorable regulatory environment.
• 2024: Government educational initiatives strengthened through seminars, clinical evidence assessments, and continuing medical education programs promoting physician confidence in prescribing biosimilars and normalizing informed switching practices.
Key Highlights of the Report
• Market Performance (2019-2024)
• Market Outlook (2025-2033)
• Industry Catalysts and Challenges
• Segment-wise historical and future forecasts
• Competitive Landscape and Key Player Analysis
• Molecule, Manufacturing Type, and Indication Analysis
Ask analyst for your customized sample:
https://www.imarcgroup.com/request?type=report&id=31900&flag=FF
Q&A Section
Q1: What drives growth in the Australia biosimilar market?
A1: Market growth is driven by government policy support through PBS listings and PBAC evaluations, healthcare cost containment priorities managing treatment expenses, clinical evidence accumulation building physician confidence, chronic disease epidemiology creating sustained biologic demand, regulatory clarity advancement facilitating market entry, and hospital procurement initiatives enabling competitive acquisition.
Q2: What are the latest trends in this market?
A2: Key trends include PBS listing acceleration with products like Wezlana receiving recommendations, clinical research leadership attracting international developers, healthcare provider acceptance growth through education and evidence, patent cliff opportunities from biologic expirations, chronic disease treatment focus requiring affordable options, and private investment surge from pharmaceutical companies.
Q3: What challenges do companies face?
A3: Major challenges include physician reluctance to switch stable patients from originators, limited patient awareness creating mistaken perceptions about biosimilars, originator brand loyalty maintaining market dominance through marketing, substitution barriers with regulatory restrictions, and manufacturing complexity requiring sophisticated capabilities and capital investment.
Q4: What opportunities are emerging?
A4: Emerging opportunities include oncology and autoimmune therapy expansion in high-cost treatment areas, hospital tender participation securing institutional contracts, local manufacturing development enhancing supply chain resilience, digital health integration improving awareness and accessibility, and physician education partnerships building prescriber confidence through CME programs.
Contact Us
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No: (D) +91-120-433-0800
United States: +1-201-971-6302
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses. IMARC's information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company's expertise.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Australia Biosimilar Market Projected to Reach USD 5,512.5 Million by 2033 here
News-ID: 4224663 • Views: …
More Releases from IMARC Group

Green Tea Bags Manufacturing Plant DPR 2026: Investment Cost, Market Growth & RO …
Setting up a green tea bags manufacturing plant positions investors within one of the steadily expanding and health-oriented segments of the global beverage industry, driven by increasing consumer awareness of wellness, rising preference for natural antioxidants, and growing demand for convenient herbal drink options. Green tea is widely valued for its perceived health benefits, including metabolism support and antioxidant properties, making it popular among health-conscious urban populations.
The shift toward…
Calcium Acetate Prices Q4 2025: USA Reaches USD 1,165/MT While China Trades at U …
North America Calcium Acetate Price Outlook Q4 2025:
United States Calcium Acetate Price Overview:
In Q4 2025, calcium acetate prices in the United States reached USD 1165 per metric ton. The market remained firm due to steady demand from food processing, pharmaceuticals, and wastewater treatment sectors. Stable consumption patterns and moderate production costs supported pricing levels. Supply chain efficiency and consistent raw material availability helped prevent sharp fluctuations during the quarter.
Get the…

Automotive Radiator Manufacturing Plant DPR & Unit Setup - 2026: Demand Analysis …
Setting up an Automotive Radiator manufacturing plant positions investors in one of the most critical and high-demand segments of the global automotive components and thermal management industry, backed by sustained global growth driven by rising vehicle production, increasing regulatory focus on engine efficiency and emission reduction, and the growing adoption of electric and hybrid vehicles requiring advanced cooling solutions. As global automotive production expands across emerging economies, regulatory frameworks continue…

Watch Manufacturing Plant DPR & Unit Setup - 2026: Machinery, CapEx/OpEx, ROI an …
Setting up a watch manufacturing plant positions investors at the convergence of precision engineering, consumer lifestyle, luxury goods, and wearable technology - one of the most dynamic and diversified segments of the global consumer goods industry - driven by rising demand for luxury and premium accessories, increasing adoption of smart and hybrid watches, growing disposable incomes across emerging markets, and expanding e-commerce and organized retail channels enabling access to global…
More Releases for Australia
Derila Memory foam pillow Australia: Honest Reviews About Derila Australia
Derila is one of the best memory foam pillows sold in Australia today.
Priced at around 30 dollars (USD), derila is currently the most reviewed and the cheapest memory pillow available in Australia.
What is Derila? Is Derila Pillow the best in Australia? Keep reading to discover everything worth knowing about Derila Australia.
OVERVIEW
Recently, Memory foam pillow has been trending and there is a lot of brands to choose from. Which one is…
CeraCare Australia - Where to Buy Legit CeraCare Supplement in Australia?
CeraCare Australia - Ceracare is a glucose support supplement that proposes to augment cardiovascular prosperity and to stay aware of perfect glucose assimilation in Australia. CeraCare supplement is conceptualized and executed by a threesome – Christine, Dr. Jihn and Michael. It is a natural supplement that helps one stay aware of ideal glucose levels, cardiovascular prosperity, and glucose assimilation.
Take Advantage of 80% Discount Offer in Australia >> https://boostsxproaustralia.com/ceracare-new
The indications…
Glucofort Australia - Where to Buy Legit Glucofort Supplement in Australia?
Glucofort Australia - Glucofort is an efficient, all-natural progressive glucose support supplement in Australia. This formula is made out of 12 key ingredients, 7 nutrients, and minerals, and a little of Vanadium. This supplement upholds regulated glucose levels and glucose digestion. Glucofort prides itself as the most inventive supplements available in Oceania, accentuating its solidarity, wellbeing, and quality.
Take Advantage of 75% Discount Offer in Australia >> https://boostsxproaustralia.com/glucofort-new
Rather than simply…
Australia Agriculture Market, Australia Agriculture Industry, Australia Agricult …
Australia Agriculture has been as vital within the development of Australia, because it was within the United States. Australia's ancient dominance in wheat and sheep continues into the 21st century. Recently Australian agriculture has become more and more diversified. The considerable expanses of productive land have helped Australia to become a number one world exporter of grains, meats, and wool. Each grains (predominantly wheat and barley) and wool markets round…
Australia Conveyor Maintenance Analysis by Top Companies Habasit Australia Pty l …
Global Australia Conveyor Maintenance Market and Competitive Analysis
Know your current market situation! Not only a vital element for brand new products but also for current products given the ever-changing market dynamics. The study allows marketers to remain involved with current consumer trends and segments where they'll face a rapid market share drop. Discover who you actually compete against within the marketplace, with Market Share Analysis know market position, to push…
Australia Conveyor Maintenance Market Analysis By Manufacturers Rema Tip Top Aus …
A conveyor system is a common piece of mechanical handling device that moves materials/objects from one location. A conveyor is often lifeline to a company’s ability to effectively move its products in a timely manner. While it is used constantly in a manufacturing plant, proper maintenance from trained technicians can extend the lifespan of conveyor. Furthermore, conveyor maintenance is essential as it may be subjected to different types of failures…