Press release
Hemorrhagic Cystitis Market Witnesses Significant Growth: Key Market Opportunities and Drivers | DelveInsight
DelveInsight's comprehensive analysis reveals robust market expansion driven by growing patient population, emerging therapeutics, and strategic industry developments across major pharmaceutical companies including Lipella Pharmaceuticals, Urigen Pharmaceuticals, Evrys Bio, and Ixaltis, among others.Key Findings on Hemorrhagic Cystitis Market
As per DelveInsight's analysis, the total Hemorrhagic Cystitis market size in the 7MM is expected to witness a major change in the study period 2019-2032, with substantial growth anticipated across the United States, EU4 countries, the UK, and Japan. Key market drivers of Hemorrhagic Cystitis include rising awareness of the disease, incremental healthcare spending across the world, growing need for better technology, increasing patient pool, and expanding cancer treatment regimens that predispose patients to this serious complication.
The report provides the total Hemorrhagic Cystitis potential pool segmented by total diagnosed cases in the 7MM and gender-specific cases across major markets, with epidemiology data spanning from 2019 to 2032. Leading Hemorrhagic Cystitis companies, such as Lipella Pharmaceuticals Inc, Urigen Pharmaceuticals Inc, Ixaltis, Evrys Bio, and others, are actively developing innovative therapeutic solutions to address this significant unmet medical need.
Some of the key Hemorrhagic Cystitis therapies in the pipeline include LP-10 (liposomal tacrolimus) from Lipella Pharmaceuticals, which has received FDA Orphan Disease Designation and demonstrated promising efficacy in Phase 2a studies. Recent developments include Lipella Pharmaceuticals' ongoing Phase 2b trial preparation for LP-10, following positive results from their dose-escalation study showing significant improvements in hematuria, bleeding sites on cystoscopy, and urinary symptoms. Additionally, the FDA approved several related therapies in 2025, including gemcitabine intravesical system (INLEXZO) by Janssen Biotech for bladder cancer, and mitomycin intravesical solution (ZUSDURI) by UroGen Pharma for recurrent bladder cancer, demonstrating increased regulatory focus on intravesical therapies.
Get comprehensive Hemorrhagic Cystitis market overview [https://www.delveinsight.com/sample-request/hemorrhagic-cystitis-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr] including key findings, competitive landscape analysis, and strategic recommendations for pharmaceutical executives.
Hemorrhagic Cystitis Market and Epidemiology Analysis
The Hemorrhagic Cystitis market represents a critical therapeutic area addressing a serious, life-threatening bladder condition caused by pelvic radiation therapy and bladder-toxic chemotherapies including cyclophosphamide and ifosfamide. Current market dynamics show substantial unmet need, as there are currently no FDA-approved drug treatments specifically for Hemorrhagic Cystitis, creating significant opportunities for pipeline therapeutics.
According to DelveInsight's comprehensive Hemorrhagic Cystitis Market Insight, Epidemiology and Market Forecast report [https://www.delveinsight.com/sample-request/hemorrhagic-cystitis-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr], the Hemorrhagic Cystitis market growth projections indicate robust expansion through 2032, driven primarily by the growing cancer survivor population receiving aggressive treatment modalities. The pelvic cancer-induced hemorrhagic cystitis market specifically is projected to grow significantly by 2032. The growth reflects the increasing incidence of pelvic malignancies and prolonged survival rates that create larger populations at risk for developing hemorrhagic cystitis complications.
DelveInisght's geographic analysis reveals the United States as the largest market, followed by EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan. The seven major markets (7MM) coverage provides comprehensive epidemiological insights spanning total diagnosed cases and gender-specific segmentation, with males typically showing higher incidence rates due to prostate cancer treatment patterns.
Additionally, epidemiological trends across the 7MM demonstrate steady growth in the patient population from 2019 through 2032, correlating with expanding cancer treatment protocols and improved survival rates. The diagnosed patient pool encompasses various severity grades, from microscopic hematuria to severe bladder hemorrhage requiring intensive medical intervention. Key risk factors include prior pelvic radiation exposure, chemotherapy history, and underlying malignancies, with bone marrow transplant recipients representing a particularly vulnerable population.
Access detailed Hemorrhagic Cystitis market forecasts, competitive intelligence, and investment opportunities [https://www.delveinsight.com/sample-request/hemorrhagic-cystitis-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr] across the 7MM through DelveInsight's specialized research platform.
Hemorrhagic Cystitis Competitive Landscape
Key Hemorrhagic Cystitis companies include Lipella Pharmaceuticals (LP-10 liposomal tacrolimus), Urigen Pharmaceuticals (developing urological treatments), Ixaltis (pipeline molecules from Sanofi), and Evrys Bio, among others. The clinical landscape currently relies on supportive care measures including hyperhydration, continuous bladder irrigation, and various intravesical therapies such as alum, aminocaproic acid, and hyperbaric oxygen therapy.
Drug profiles reveal LP-10 as the most advanced pipeline asset, utilizing a liposomal tacrolimus formulation for intravesical administration. The mechanism of action involves local immunomodulation to reduce bladder inflammation and bleeding, with Phase 2a studies demonstrating dose-dependent improvements in multiple efficacy measures. Development milestones include successful completion of Phase 2a dose-escalation trials, FDA Orphan Disease Designation, and ongoing preparation for Phase 2b registration-enabling studies.
Recent trial results from the RICH-ART study demonstrate long-term benefits of hyperbaric oxygen therapy, with 5-year follow-up data showing sustained symptom reduction in chronic radiation-induced cystitis patients. The Hemorrhagic Cystitis clinical trial activity includes ongoing Phase II studies evaluating novel approaches such as oral chlorophyllin, showing promising results in radiation-induced hemorrhagic cystitis.
Commercial arrangements include Lipella Pharmaceuticals' FDA Type C meeting discussions for Phase 2b trial design optimization. Strategic collaborations focus on addressing manufacturing scalability and regulatory pathway development, particularly for orphan drug designations that provide market exclusivity incentives.
Pipeline developments encompass five active therapeutic programs, with three in preclinical stages and advancing through clinical development phases. Key targets include sirtuin and calcineurin pathways, representing novel mechanisms for controlling bladder inflammation and bleeding.
Discover emerging Hemorrhagic Cystitis treatments, clinical trial updates, and regulatory milestones to inform strategic planning and partnership decisions. [https://www.delveinsight.com/sample-request/hemorrhagic-cystitis-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr]
Hemorrhagic Cystitis Market Drivers and Barriers
Unmet medical needs represent the primary market driver, as current management relies primarily on supportive care without specific FDA-approved therapeutic interventions. The lack of effective treatments creates significant clinical challenges, particularly for patients with refractory disease requiring invasive procedures such as cystectomy with urinary diversion.
Additional drivers include the expanding cancer treatment population, with approximately 20% of pelvic cancer patients receiving radiation therapy that increases hemorrhagic cystitis risk. Growing awareness among healthcare providers and improved diagnostic capabilities contribute to better patient identification and treatment initiation. The orphan drug designation pathway provides regulatory incentives for companies developing treatments for this rare condition.
Market barriers include the complex pathophysiology of hemorrhagic cystitis, which involves multiple inflammatory pathways making drug development challenging. Limited patient populations for clinical trials create recruitment difficulties, while the serious nature of the condition requires careful safety monitoring throughout development programs. Regulatory requirements for demonstrating clinical benefit in a heterogeneous patient population with varying disease severity present additional hurdles.
The Hemorrhagic Cystitis competitive landscape remains fragmented, with most therapies in early development phases, creating uncertainty about commercial viability and market penetration. Healthcare reimbursement considerations for orphan drugs may limit patient access despite clinical benefits, particularly in resource-constrained healthcare systems.
Obtain detailed Hemorrhagic Cystitis patient population data, demographic segmentation, and marekt drivers/barriers [https://www.delveinsight.com/sample-request/hemorrhagic-cystitis-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=xpr] for market sizing and commercial strategy development.
Conclusion
The Hemorrhagic Cystitis market stands at a critical inflection point, with promising pipeline therapeutics advancing toward commercialization amid substantial unmet medical need. DelveInsight's analysis indicates significant market expansion potential through 2032, driven by growing patient populations, innovative drug development programs, and supportive regulatory environments for orphan diseases. Key companies including Lipella Pharmaceuticals are positioned to capture substantial market share with first-in-class therapeutic approaches, while continued clinical research and strategic partnerships will likely accelerate treatment availability for this underserved patient population.
Key Opinion Leader Perspective: Leading urologists emphasize the critical need for effective Hemorrhagic Cystitis treatments, noting that current management approaches remain largely supportive despite advances in cancer therapy that increase patient risk profiles.
DelveInsight's Analysis: The Hemorrhagic Cystitis market represents a high-value opportunity with substantial barriers to entry, favorable orphan drug regulatory pathways, and significant first-mover advantages for companies successfully navigating clinical development challenges.
Scope of the Report
*
The report covers the descriptive overview of Hemorrhagic Cystitis, explaining its causes, signs and symptoms, pathophysiology, diagnosis and currently available therapies
*
Comprehensive insight has been provided into the Hemorrhagic Cystitis epidemiology and treatment in the 7MM
*
Additionally, an all-inclusive account of both the current and emerging therapies for Hemorrhagic Cystitis is provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape
*
A detailed review of the Hemorrhagic Cystitis market; historical and forecasted is included in the report, covering drug outreach in the 7MM
*
The report provides an edge while developing business strategies, by understanding trends shaping and driving the global Hemorrhagic Cystitis market
*
Our in-depth analysis of the pipeline assets across different stages of development (Phase III and Phase II), different emerging trends and comparative analysis of pipeline products with detailed clinical profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process
Table of Contents
1. Key Insights
2. Executive Summary of Hemorrhagic Cystitis
3. Competitive Intelligence Analysis for Hemorrhagic Cystitis
4. Hemorrhagic Cystitis Market Overview at a Glance
5. Hemorrhagic Cystitis: Disease Background and Overview
6. Hemorrhagic Cystitis Patient Journey
7. Hemorrhagic Cystitis Epidemiology and Patient Population
8. Treatment Algorithm, Current Treatment, and Medical Practices
9. Hemorrhagic Cystitis Unmet Needs
10. Key Endpoints of Hemorrhagic Cystitis Treatment
11. Hemorrhagic Cystitis Marketed Products
12. Hemorrhagic Cystitis Emerging Therapies
13. Hemorrhagic Cystitis: Seven Major Market Analysis
14. Attribute analysis
15. 7MM: Market Outlook
16. Access and Reimbursement Overview of Hemorrhagic Cystitis
17. KOL Views
18. Hemorrhagic Cystitis Market Drivers
19. Hemorrhagic Cystitis Market Barriers
20. Appendix
21. DelveInsight Capabilities
22. Disclaimer
23. About DelveInsight
About DelveInsight
DelveInsight is a leading market research and consulting firm specializing in disease-specific insights and therapeutic market analysis. Their reports integrate real-world data, clinical trial findings, and expert interviews to deliver comprehensive industry intelligence.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Arpit Anand
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=hemorrhagic-cystitis-market-witnesses-significant-growth-key-market-opportunities-and-drivers-delveinsight]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/consulting/due-diligence-services
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Hemorrhagic Cystitis Market Witnesses Significant Growth: Key Market Opportunities and Drivers | DelveInsight here
News-ID: 4221698 • Views: …
More Releases from ABNewswire

Windy City Chimney Heroes Announces Expanded Service Areas Across the Greater Ch …
Windy City Chimney Heroes, a long-standing chimney company established in 1997, has expanded its service areas across the Greater Chicago region. The company now serves Chicago, Evanston, Oak Park, Naperville, Schaumburg, Cicero, Skokie, Berwyn, and Arlington Heights. Owner Wesley Cook says the expansion will help meet rising demand for reliable chimney repair and inspection services.
Windy City Chimney Heroes, a trusted chimney repair and maintenance provider founded in 1997, has announced…

Injury 2 Wellness Centers Promotes Wellness Care Through Ongoing Chiropractic Su …
As the year draws to a close and many individuals begin setting health goals for the new year, Injury 2 Wellness Centers is encouraging Georgia residents to prioritize wellness care through consistent chiropractic treatment. Far beyond addressing back or neck pain, chiropractic care can serve as a foundation for long-term physical health, improved function, and overall quality of life.
Decatur, GA - December 10, 2025 - As the year draws to…

Avalon Tree Services Reminds Atlanta Homeowners to Prioritize Winter Tree Care f …
As temperatures drop and trees enter their dormant season, Avalon Tree Services is reminding homeowners across Greater Atlanta that winter is one of the most important times to invest in proper tree care. Cold weather, ice accumulation, and seasonal storms can all take a toll on tree health and stability-making preventative maintenance essential for safety and long-term growth.
Atlanta, GA - December 10, 2025 - As temperatures drop and trees enter…
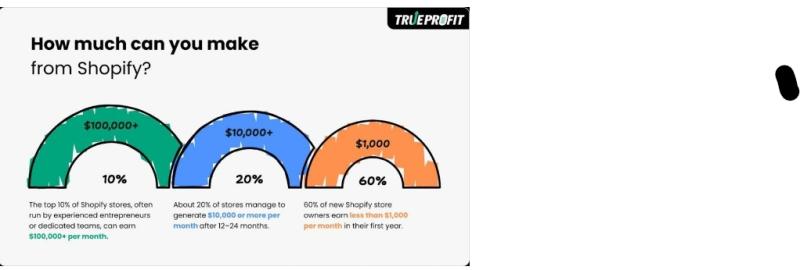
TrueProfit Shares 2026 Guidance for Shopify Startups to Focus on Profit First No …
As Shopify heads into 2026 with millions of active stores worldwide and continued growth in global ecommerce, TrueProfit - a Net Profit Analytics platform for Shopify merchants - is urging new founders to treat profitability as a starting requirement, not an afterthought.
With a low barrier to entry and a mature app ecosystem, Shopify remains one of the easiest ways to launch an online business. But TrueProfit's latest briefing on early-stage…
More Releases for Hemorrhagic
Hemorrhagic Shock Treatment Market Deep Research Report - CSL Behring, Octapharm …
Hemorrhagic Shock Treatment Market Insights
Hemorrhagic Shock Treatment Market is estimated to be valued at USD 224.6 Mn in 2025 and is expected to reach USD 303.6 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.4% from 2025 to 2032.
Coherent Market Insights has published a new comprehensive analysis on the Hemorrhagic Shock Treatment Market (2025-2032), focusing on critical transformations in the U.S. healthcare environment. This report highlights essential…
Alkhurma Hemorrhagic Fever Treatment Market
Alkhurma Hemorrhagic Fever (AHF) is a viral disease that primarily affects humans and is transmitted by ticks, specifically the Hyalomma species. First identified in Saudi Arabia in 1994, AHF is characterized by severe symptoms, including fever, hemorrhage, and multi-organ failure. The absence of specific antiviral treatments makes it crucial to develop effective therapeutic options. As awareness of the disease grows, so does the demand for innovative treatments, leading to the…
Complete Viral Hemorrhagic Fever Treatment Market Analysis & Forecast 2023-2031
𝐆𝐫𝐨𝐰𝐭𝐡 𝐌𝐚𝐫𝐤𝐞𝐭 𝐑𝐞𝐩𝐨𝐫𝐭𝐬 has included a latest report on the Global Viral Hemorrhagic Fever Treatment Market into its archive of market research studies. The report is an amalgamation of detailed market overview based on the segmentations, applications, trends and opportunities, mergers and acquisitions, drivers, and restraints.
The report showcases the current and forthcoming technical and financial details of the Viral Hemorrhagic Fever Treatment market. The research study attracts attention to…
Hemorrhagic Stroke Treatment Market Research Insights Shared in Detailed Report
Hemorrhagic stroke is also called cerebral hemorrhage. It is a condition caused by the rapture and bleeding of cerebral blood vessels. There are two types of hemorrhagic stroke, intracerebral (within the brain) hemorrhage and subarachnoid hemorrhage. Hemorrhagic stroke occurs when a weakened blood vessel inside the brain bursts and leaks blood into surrounding brain tissue (intracerebal hemorrhage). Two types of weakened blood vessels usually cause hemorrhagic stroke, aneurysms and arteriovenous…
Viral Hemorrhagic Fever Market: Segmentation, Global Market Regional Outlook 202 …
Viral Hemorrhagic Fever Market: Overview
Viral hemorrhagic fever is referred to as severe multisystem (multiple organs) syndrome in our body that is affected by certain viruses. Viral hemorrhagic fever can be described as a group of illness caused by four families of RNA viruses namely arenaviruses, filoviruses, bunyaviruses and flaviviruses. Due to hemorrhagic fever, the overall vascular system in our body gets damaged and the body loses its ability to regulate.…
Report delivers insight into the Hemorrhagic Shock-Pipeline Insights, 2017
"The Report Hemorrhagic Shock-Pipeline Insights, 2017 provides information on pricing, market analysis, shares, forecast, and company profiles for key industry participants. - MarketResearchReports.biz"
DelveInsights, Hemorrhagic Shock-Pipeline Insights, 2017, report provides in depth insights on the pipeline drugs and their development activities around the Hemorrhagic Shock. The DelveInsightsReport covers the product profiles in various stages of development including Discovery, Pre-clinical, IND, Phase I, Phase II, Phase III and Preregistration. Report covers…
