Press release
Future of Duchenne Muscular Dystrophy Treatment Market Research Report: Latest Treatments, Trials & Industry Insights | Most Leading Companies - Sarepta Therapeutics, Inc., ITF Therapeutics LLC, NS Pharma, Inc., Catalyst Pharmaceuticals, Inc., and PTC The
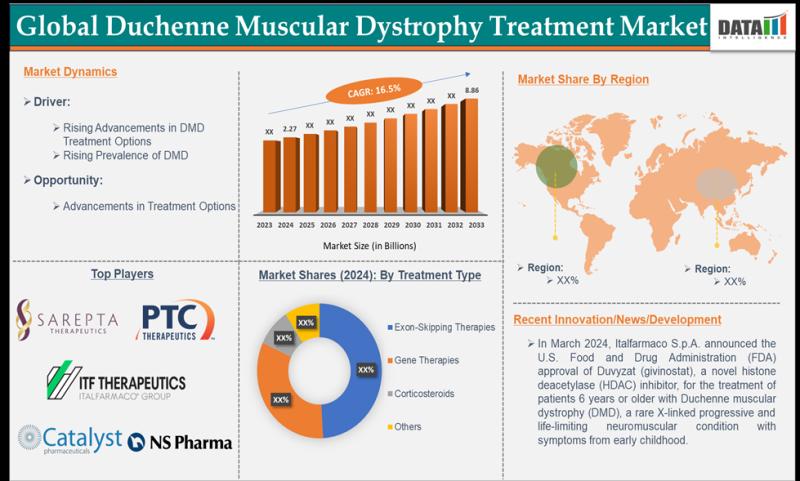
Duchenne muscular dystrophy treatment market size reached US$ 2.27 billion in 2024 and is expected to reach US$ 8.86 billion by 2033, growing at a CAGR of 16.5% during the forecast period 2025-2033.DataM Intelligence has published a new research report on "Global Duchenne Muscular Dystrophy Treatment Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Elevidys, an FDA-approved gene therapy, has a list price of around $3.2 million per patient, ranking it among the most costly treatments ever developed. Other Duchenne muscular dystrophy (DMD) therapies, including Exondys 51 and Vyondys 53, also carry annual costs exceeding $300,000, although their effectiveness can vary based on the patient's specific genetic mutation.
Gene therapies such as Elevidys (delandistrogene moxeparvovec) and upcoming pipeline candidates like RGX-202, SGT-003, and GNT0004 aim to deliver a functional version of the dystrophin gene to muscle cells, either through direct gene delivery or advanced gene-editing technologies.
RGX-202 from REGENXBIO is anticipated to gain approval in 2027, while SGT-003 from Solid Biosciences is expected by 2026. These emerging gene therapies are set to reshape the market in the coming years, offering improved treatment options for patients.
Get a Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://datamintelligence.com/download-sample/duchenne-muscular-dystrophy-treatment-market?kb
United States: Regulatory and Clinical Advancements
✅ FDA Greenlights Elevidys for Ambulatory Patients
In August 2025, the FDA recommended resuming shipments of Sarepta Therapeutics' gene therapy, Elevidys, for ambulatory DMD patients aged 4 and older. This decision followed a thorough investigation into previous safety concerns, including acute liver injury cases in non-ambulatory patients. Elevidys remains the first FDA-approved gene therapy for DMD.
✅ Breakthrough Therapy Designation for DYNE-251
Dyne Therapeutics received FDA Breakthrough Therapy Designation for DYNE-251, an investigational RNA-based therapy targeting exon 51 mutations. Early clinical data from the DELIVER trial indicate sustained functional improvements, such as faster time to rise and improved walking speed over 18 months.
✅ Duvyzat (Givinostat) Approved for All DMD Variants
The FDA approved Duvyzat, a non-steroidal drug by Italfarmaco, for DMD patients aged 6 and older. Unlike exon-skipping therapies, Duvyzat aims to reduce muscle damage and inflammation, offering a treatment option for all genetic variants of DMD.
Japan: Conditional Approvals and Ongoing Trials
✅ Elevidys Receives Conditional Approval
In May 2025, Elevidys was conditionally approved in Japan for children aged 3-7 without deletions in exons 8 and/or 9 of the DMD gene. The approval is valid for up to seven years, pending further clinical data to confirm long-term safety and efficacy.
✅ Taiho's TAS-205 Fails Phase III Endpoint
Taiho Pharmaceutical's Phase III study of TAS-205, an oral therapeutic for ambulatory DMD patients, did not meet its primary endpoint of improving time to rise from the floor. The study enrolled 82 patients across 26 sites in Japan.
✅ Orphan Drug Designation for NS-051/NCNP-04
The FDA granted Orphan Drug Designation to NS-051/NCNP-04, an exon 51 skipping drug developed by the National Center of Neurology and Psychiatry (NCNP) in Japan. This designation supports the development of therapies for rare diseases.
South Korea: Evolving Landscape and Challenges
✅ Gene Therapy Faces Setbacks
Clinical trials in South Korea have highlighted challenges with gene therapies targeting DMD, leading to a reevaluation of their efficacy and safety. As a result, alternative treatment approaches are gaining traction in the country.
✅ Emerging Therapeutics Market
The South Korean DMD therapeutics market is undergoing rapid transformation, driven by technological innovation and increasing investment in research and development. This shift is expected to lead to the introduction of new treatment options in the near future
Key Players:
Sarepta Therapeutics, Inc., ITF Therapeutics LLC, NS Pharma, Inc., Catalyst Pharmaceuticals, Inc., and PTC Therapeutics.
Emerging players in the market include F. Hoffmann-La Roche Ltd, Capricor Therapeutics, Inc., REGENXBIO Inc., Solid Biosciences Inc., Wave Life Sciences, Genethon
Key Development:
In January 2025, Roche reported positive year-two results from the EMBARK phase III trial of Elevidys (delandistrogene moxeparvovec), showing sustained functional improvements in Duchenne muscular dystrophy (DMD) patients compared with external controls.
In June 2024, Sarepta expanded the FDA-approved indication for Elevidys to include DMD patients aged 4 and above with confirmed DMD gene mutations. The FDA granted traditional approval for ambulatory patients and accelerated approval for non-ambulatory patients, with continued approval contingent on confirmatory trials. Elevidys is contraindicated in patients with deletions in exon 8 and/or 9.
In March 2024, Italfarmaco received FDA approval for Duvyzat (givinostat), a novel HDAC inhibitor, for DMD patients aged 6 years and older, offering a new treatment option for this rare, progressive neuromuscular disorder.
Growth Forecast Projected:
The Global Global Duchenne Muscular Dystrophy Treatment Market is anticipated to rise at a considerable rate during the forecast period, between 2025 and 2032. In 2024, the market is growing at a steady rate, and with the rising adoption of strategies by key players, the market is expected to rise over the projected horizon.
Research Process:
Both primary and secondary data sources have been used in the global Global Duchenne Muscular Dystrophy Treatment Market research report. During the research process, a wide range of industry-affecting factors are examined, including governmental regulations, market conditions, competitive levels, historical data, market situation, technological advancements, upcoming developments, in related businesses, as well as market volatility, prospects, potential barriers, and challenges.
Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=duchenne-muscular-dystrophy-treatment-market?kb
Key Segments: CCC
Regional Analysis for Market:
⇥ North America (U.S., Canada, Mexico)
⇥ Europe (U.K., Italy, Germany, Russia, France, Spain, The Netherlands and Rest of Europe)
⇥ Asia-Pacific (India, Japan, China, South Korea, Australia, Indonesia Rest of Asia Pacific)
⇥ South America (Colombia, Brazil, Argentina, Rest of South America)
⇥ Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of Middle East & Africa)
Benefits of the Report:
Chapter 1: Sets the stage by outlining the report's coverage, summarizing key market segments by region, product type, and application. Presents a snapshot of market sizes, growth potential across segments, and anticipated industry evolution both short and long term.
Chapter 2: Highlights pivotal market insights and uncovers the most significant emerging trends driving change within the industry.
Chapter 3: Offers an in-depth look at the competitive landscape among Global Duchenne Muscular Dystrophy Treatment producers, including revenue shares, strategic moves, and recent mergers and acquisitions.
Chapter 4: Presents comprehensive profiles of the market's key players, delving into details such as revenue, profit margins, product portfolios, and company milestones.
Chapters 5 & 6: Analyze Global Duchenne Muscular Dystrophy Treatment revenue at both regional and country levels, providing quantitative breakdowns of market sizes, growth opportunities, and development prospects worldwide.
Chapter 7: Focuses on different market segments by type, examining their individual sizes and potential, guiding readers toward high-impact, untapped market areas.
Chapter 8: Explores segmentation by application, evaluating industry growth potential in various downstream markets and pinpointing promising sectors for expansion.
Chapter 9: Provides a thorough review of the industry's supply chain mapping out both upstream and downstream activities.
Chapter 10: Concludes with a summary of the report's key findings and highlights the most critical takeaways for industry stakeholders.
Get Customization in the report as per your requirements: https://datamintelligence.com/customize/duchenne-muscular-dystrophy-treatment-market?kb
FAQ's
Q1: How fast is the Global Duchenne Muscular Dystrophy Treatment Market growing?
A: The Market is on an impressive growth trajectory, expected to expand at a CAGR of 16.5% from 2025 to 2032
Q2: Which regions are dominating the Global Duchenne Muscular Dystrophy Treatment Market and which are fastest-growing?
A: North America dominating the Global Duchenne Muscular Dystrophy Treatment market.
Have any Query? Talk to our Expert @ https://www.datamintelligence.com/enquiry/duchenne-muscular-dystrophy-treatment-market?kb
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Future of Duchenne Muscular Dystrophy Treatment Market Research Report: Latest Treatments, Trials & Industry Insights | Most Leading Companies - Sarepta Therapeutics, Inc., ITF Therapeutics LLC, NS Pharma, Inc., Catalyst Pharmaceuticals, Inc., and PTC The here
News-ID: 4220928 • Views: …
More Releases from DataM Intelligence 4 Market Research LLP

Artificial Intelligence (AI) Chip Market (2026-2033) | UAE's 4 Trillion Transist …
DataM Intelligence has published a new research report on "Artificial Intelligence (AI) Chip Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Latest M & A
• Intel moves…

Japan Nurse Call Systems Market (2025-2033) | Opportunities in Hospitals and Sen …
DataM Intelligence has published a new research report on "Japan Nurse Call Systems Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Get a Sample PDF Of This…
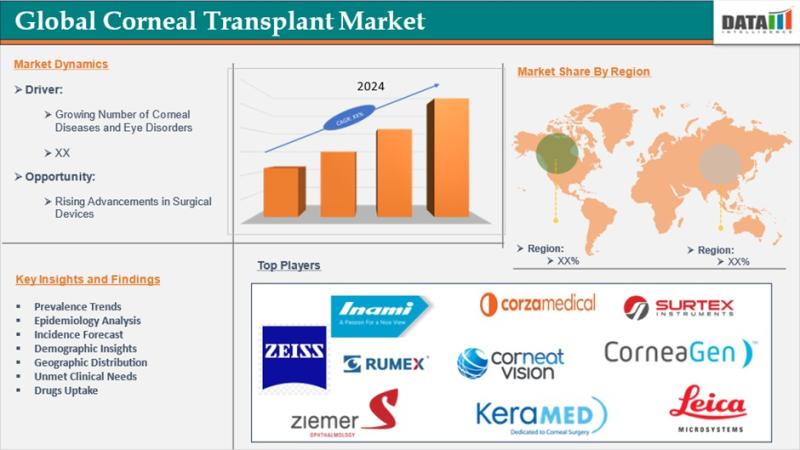
Corneal Transplant Market to Reach US$ 1,006.95 Million by 2033 at 7.59% CAGR | …
Corneal Transplant Market reached US$ 523.88 million in 2024 and is expected to reach US$ 1,006.95 million by 2033, growing at a CAGR of 7.59% during the forecast period 2025 to 2033.
Corneal transplantation, also known as keratoplasty, is a surgical procedure in which a damaged or diseased cornea is replaced with healthy donor tissue to restore vision and maintain ocular integrity. The cornea serves as the transparent outer layer of…
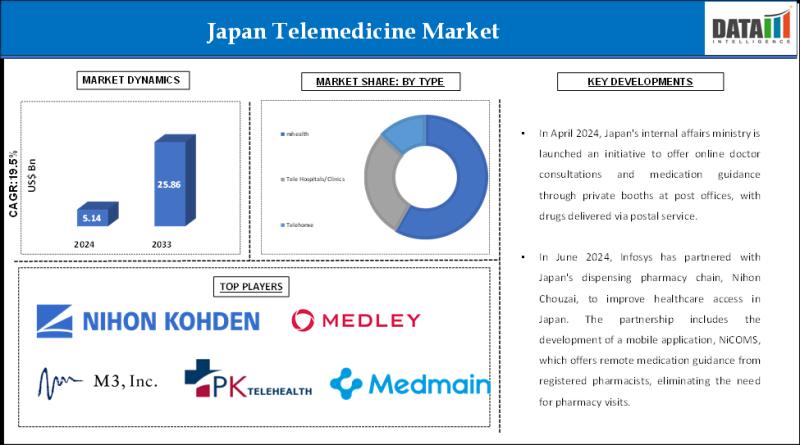
Japan Telemedicine Industry (2025-2033) | Technology Advancements and Market Pen …
DataM Intelligence has published a new research report on "Japan Telemedicine Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Get a Sample PDF Of This Report (Get…
More Releases for Duchenne
Duchenne Muscular Dystrophy (DMD) Market Growth in 2034
Market Overview
The Duchenne Muscular Dystrophy (DMD) Market is expanding rapidly as advances in genetic medicine, exon-skipping therapies, gene therapy platforms, and improved diagnostic capabilities reshape treatment options for this severe, progressive neuromuscular disorder.
DMD is caused by mutations in the dystrophin gene, leading to muscle degeneration beginning in early childhood. Growing awareness among clinicians and caregivers, widespread adoption of next-generation sequencing (NGS), and increasing availability of disease-modifying therapies have significantly strengthened…
Duchenne Muscular Dystrophy: Core Growth Enabler in the Rising Prevalence Of Chr …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
What Will the Duchenne Muscular Dystrophy Industry Market Size Be by 2025?
The market size for Duchenne Muscular Dystrophy has shown significant expansion recently, burgeoning from $1.16 billion in 2024 to $1.25 billion in 2025 at a compound annual growth rate (CAGR) of 7.9%. The impressive growth in the…
Shaping the Duchenne Muscular Dystrophy (DMD) Therapeutics Market in 2025: Bit B …
How Big Is the Duchenne Muscular Dystrophy (DMD) Therapeutics Market Expected to Be, and What Will Its Growth Rate Be?
The Duchenne muscular dystrophy (DMD) therapeutics market will grow from $11.95 billion in 2024 to $16.45 billion in 2025, at a CAGR of 37.6%. The growth is attributed to the increasing prevalence of Duchenne muscular dystrophy, rising awareness of treatment options, healthcare spending, and government initiatives.
The Duchenne muscular dystrophy (DMD) therapeutics…
Duchenne Muscular Dystrophy Pipeline Outlook Report 2024
DelveInsight's, "Duchenne Muscular Dystrophy Pipeline Insight 2024" report provides comprehensive insights about 75+ companies and 75+ pipeline drugs in the Duchenne Muscular Dystrophy pipeline landscape. It covers the Duchenne Muscular Dystrophy pipeline drug profiles, including Duchenne Muscular Dystrophy clinical trials and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
…
Duchenne Muscular Dystrophy Treatmcent Market
Global Duchenne Muscular Dystrophy Treatmcent Market Set for Robust Growth During Forecast Period
The global Duchenne Muscular Dystrophy Treatment Market is poised to witness significant growth at a high Compound Annual Growth Rate (CAGR) during the forecast period of 2023 to 2030. Duchenne muscular dystrophy (DMD) stands as a genetic disorder marked by progressive muscle degeneration and weakness, with therapeutics aimed at addressing the absence of dystrophin, a crucial protein in…
Duchenne Muscular Dystrophy (DMD) Drugs Market Report
As per the research conducted by GME, the Duchenne Muscular Dystrophy (DMD) Drugs Market will grow with a CAGR value of 41.3 percent by 2026. The global market for Duchenne muscular dystrophy is foreseen to grow due to accelerated research and development activities, expanded patient awareness for effective treatments, and the launch of new medications Moreover, the disease's increasing prominence is likely to drive the global duchenne muscular dystrophy market…